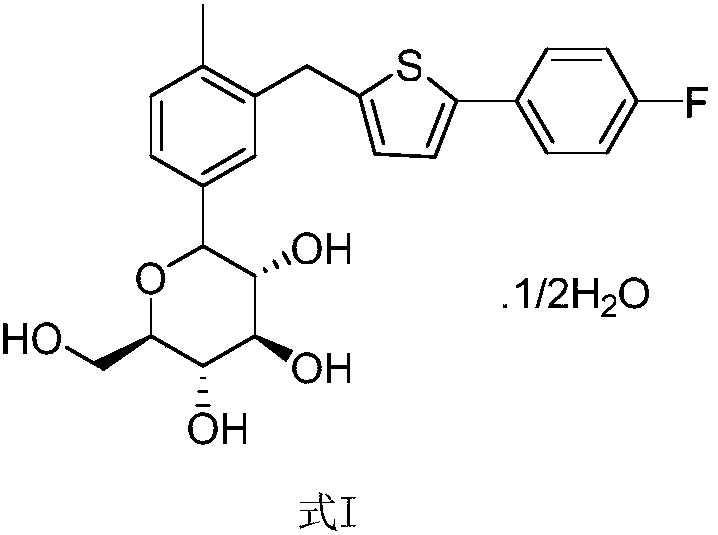
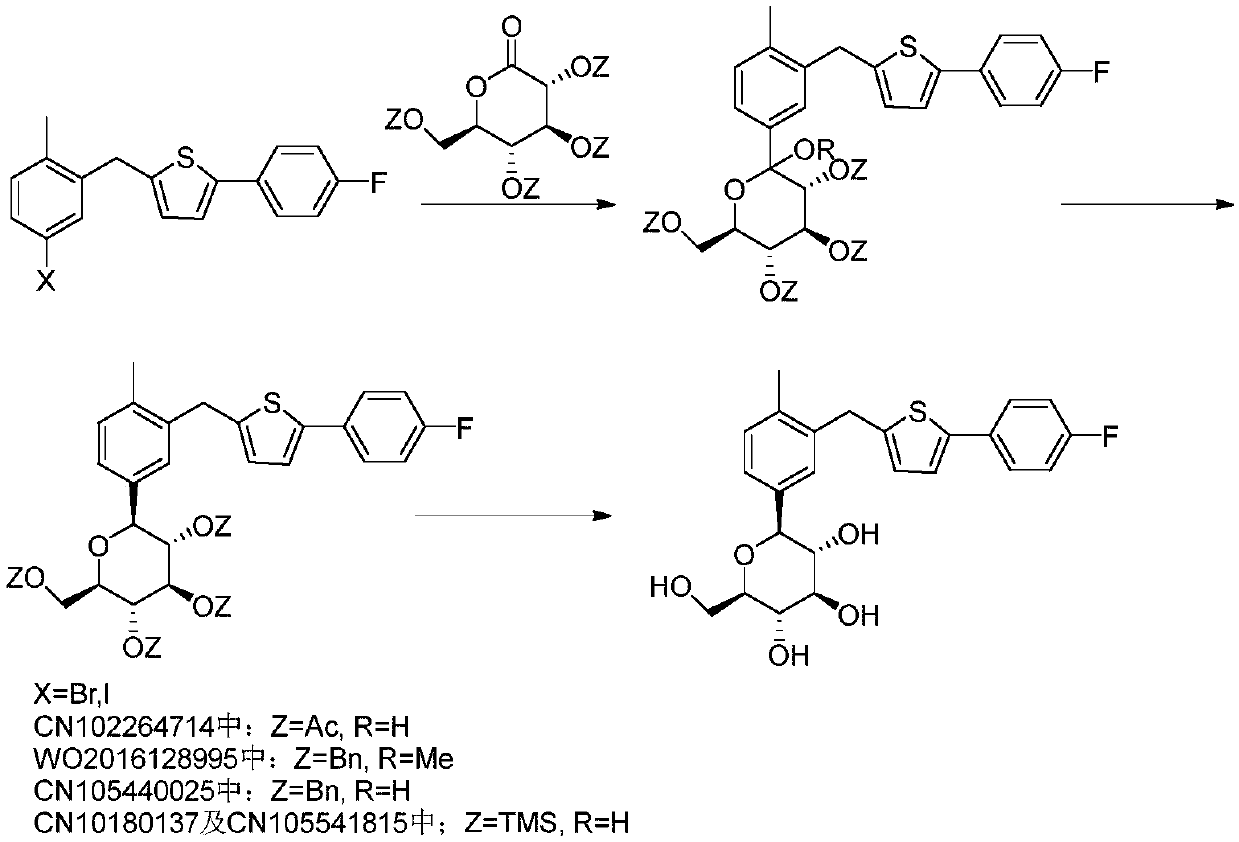
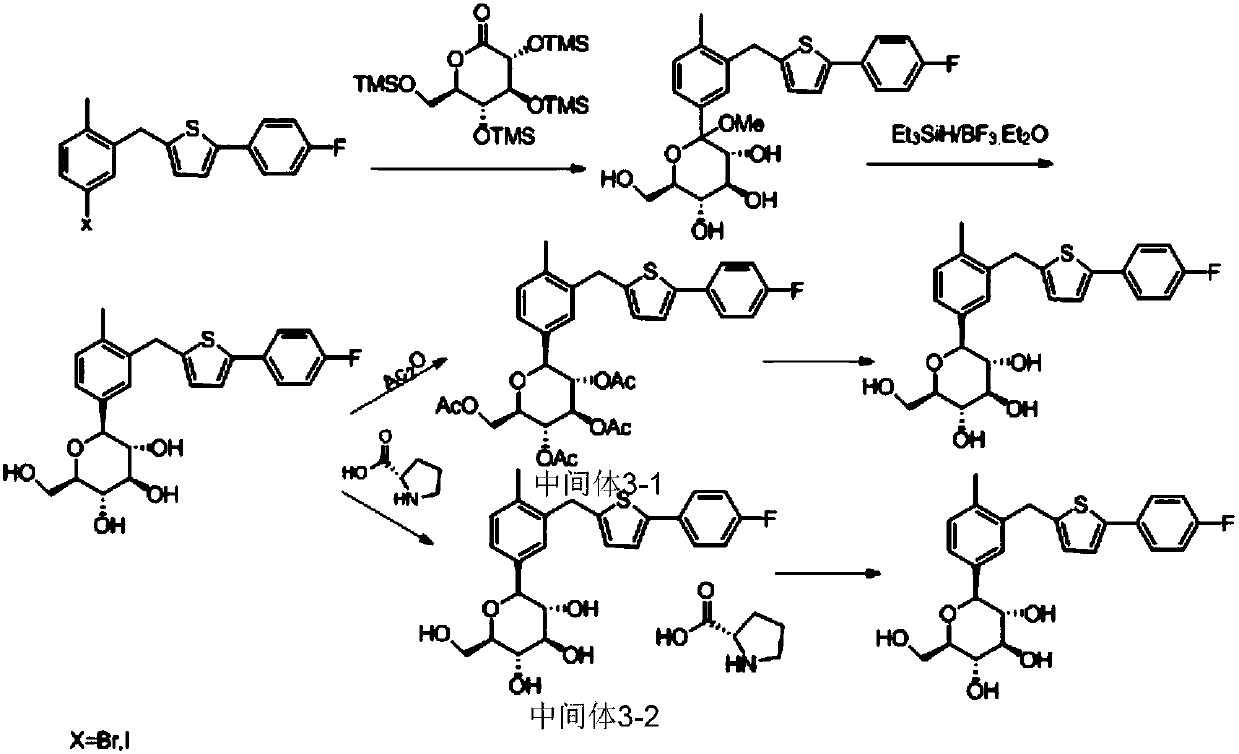
Preparation method of canagliflozin
An intermediate and molar ratio technology, applied in the field of pharmaceutical chemical synthesis, can solve the problems of low yield, high equipment requirements, and high operational safety requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091]The present embodiment is a kind of preparation method of canagliflozin, and described preparation method comprises the following steps:
[0092] 1. Preparation of Intermediate 1:
[0093] Take a 3L three-neck flask, install a mechanical stirrer, a thermometer and a constant pressure low liquid funnel; add 300ml of anhydrous tetrahydrofuran, add 100g (0.245mol) 2-(4-fluorophenyl)-5-[(5-iodo-2- Methylphenyl)methyl]thiophene, stir until dissolved; under nitrogen protection, cool down to -25~-20°C, add 263.8mL (0.343mol) sec-butylmagnesium chloride-lithium chloride / THF solution dropwise, add dropwise Complete, continue to react for 1 hour;
[0094] Maintain the temperature, add dropwise 171.6g (0.368mol) 2,3,4,6-tetra-O-(trimethylsilyl)-D-gluconolactone pre-cooled to -25~-20°C Add to the reaction solution, wherein the 2,3,4,6-tetra-O-(trimethylsilyl)-D-gluconolactone is dissolved in 200 mL of anhydrous tetrahydrofuran solution. After the dropwise addition, continue to re...
Embodiment 2
[0106] This embodiment is another preparation method of canagliflozin, the preparation method comprising the following steps:
[0107] 1. Preparation of intermediate 1:
[0108] Take a 2L three-necked flask, install a mechanical stirrer, a thermometer and a constant pressure low liquid funnel; add 150ml of anhydrous tetrahydrofuran, add 50g (0.122mol) 2-(4-fluorophenyl)-5-[(5-iodo-2- Methylphenyl)methyl]thiophene, stirred until dissolved; under nitrogen protection, and cooled to -25~-20°C, 122.5mL (0.159mol) of isopropylmagnesium chloride. Lithium chloride / THF solution was added dropwise, and the dropwise addition was completed After that, continue to react for 1 hour;
[0109] Maintain the temperature, drop 74.3g (0.159mol) of 2,3,4,6-tetra-O-(trimethylsilyl)-D-gluconolactone pre-cooled to -25~-20°C Add to the reaction solution, wherein the 2,3,4,6-tetra-O-(trimethylsilyl)-D-gluconolactone is dissolved in 100 mL of anhydrous tetrahydrofuran solution. After the dropwise add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com