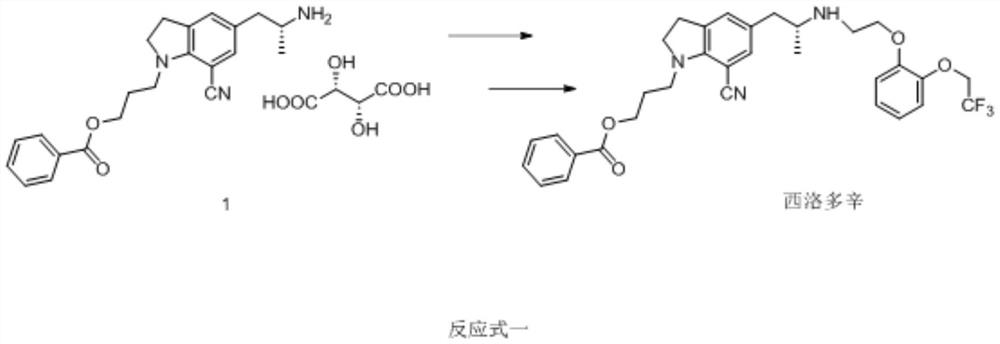
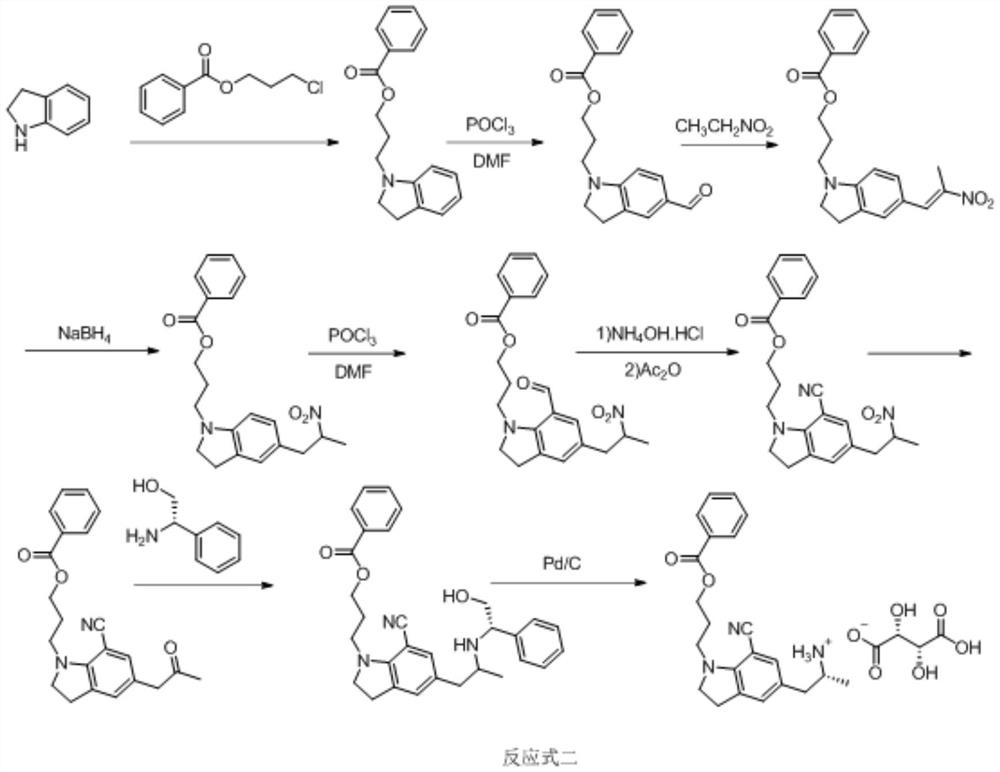
A kind of preparation method of silodosin intermediate
A technology for silodosin and intermediates, which is applied in the field of pharmaceutical preparation, can solve the problems of potential safety hazards, serious production pollution, and low total route yields in industrial production, and achieves stable properties of intermediate substances, low equipment requirements, and high yields. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] refer to figure 1 , the invention provides a kind of preparation method of silodosin intermediate, described method comprises the steps:
[0103] S1, carry out alkylation reaction with 5-bromoindoline solution and triethylamine to obtain substance A;
[0104] S2, the solution of substance A, ethyl acetoacetate, catalyst, acid binding agent are carried out substitution reaction to obtain substance B;
[0105] S3, carry out decarboxylation reaction of the solution of substance B and Lewis acid to obtain substance C;
[0106] S4, carry out amination reaction of the solution of substance C, R-tert-butylsulfinamide, catalyst, and reducing agent to obtain substance D;
[0107] S5, the solution of substance D, N-bromosuccinimide solution is carried out bromination reaction to obtain substance E;
[0108] S6. The solution of substance E and cuprous cyanide are subjected to cyanation reaction to obtain substance F;
[0109] S7, subject the solution of substance F and acid to...
Embodiment 1
[0113] A kind of preparation method of silodosin intermediate, described method comprises the steps:
[0114] S1, carry out alkylation reaction with 5-bromoindoline solution and triethylamine to obtain substance A;
[0115] S2, the solution of substance A, ethyl acetoacetate, catalyst, acid binding agent are carried out substitution reaction to obtain substance B;
[0116] S3, carry out decarboxylation reaction of the solution of substance B and Lewis acid to obtain substance C;
[0117] S4, carry out the amination reaction of the solution of substance C, R-tert-butyl sulfenamide, catalyst, and sodium borohydride solution to obtain substance D;
[0118] S5, the solution of substance D, N-bromosuccinimide solution is carried out bromination reaction to obtain substance E;
[0119] S6. The solution of substance E and cuprous cyanide are subjected to cyanation reaction to obtain substance F;
[0120] S7, subject the solution of substance F and acid to acidolysis to obtain subs...
Embodiment 2
[0123] A kind of preparation method of silodosin intermediate, described method comprises the steps:
[0124] S1, carry out alkylation reaction with 5-bromoindoline solution and triethylamine to obtain substance A;
[0125] S2, the solution of substance A, ethyl acetoacetate, catalyst, acid binding agent are carried out substitution reaction to obtain substance B;
[0126] S3, carry out decarboxylation reaction of the solution of substance B and Lewis acid to obtain substance C;
[0127] S4, carry out amination reaction of the solution of substance C, R-tert-butylsulfinamide, catalyst, and reducing agent to obtain substance D;
[0128] S5, the solution of substance D, N-bromosuccinimide solution is carried out bromination reaction to obtain substance E;
[0129] S6. The solution of substance E and cuprous cyanide are subjected to cyanation reaction to obtain substance F;
[0130] S7, subject the solution of substance F and acid to acidolysis to obtain substance G;
[0131]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com