Novel bh3 mimetic peptide compound targeting ptp1b and its preparation method and application
A technology of compound and mimetic peptide, which is applied in the field of new BH3 mimetic compound and its preparation, can solve the problem of compound difficulty and achieve the effect of excellent development prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. The specific preparation process of Pal-PUMA is as follows:
[0036] (1) Resin activation: at room temperature, weigh the corresponding amount of Fmoc-Phe-Wang resin, wash it with dichloromethane (DCM) 4 times, place it in a manual polypeptide solid-phase synthesizer, add 5ml DCM to swell and activate it for 3 hours, and dimethicone Washed 4 times with methyl formamide (DMF), added 20% piperidine DMF to remove the Fmoc protecting group for 20 min, washed 4 times with 5ml DMF, washed 4 times with 5ml DCM, and tested with Kaiser's reagent.
[0037] (2) Connecting Leu(L): DMF was washed 3 times, Fmoc-Leu-OH, HBTU, HOBt and 6 times the resin molar weight of DIEA were added respectively, dissolved in 10ml DMF, stirred at room temperature for 2 hours , washed 4 times with DMF, added 20% piperidine DMF to remove the Fmoc protecting group for 20 min, washed 4 times with 5ml DMF, washed 4 times with 5ml DCM, and detected by Kaiser's reagent.
[0038] (3) Asp(D) connection: w...
Embodiment 2
[0053] The mass spectrum data and HPLC purity analysis data of the eight BH3 mimetic peptide compounds are shown in Table 1.
[0054] Table 1. Mass Spectrometry Data and HPLC Purity Analysis Data of BH3 Mimetic Peptides
[0055]
Embodiment 3
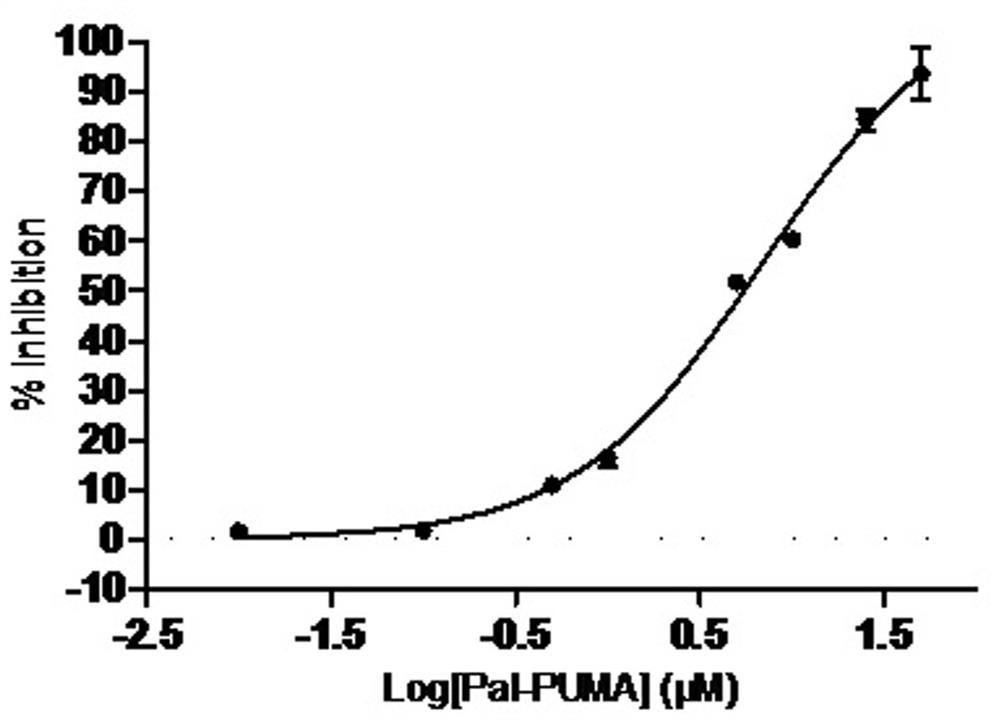
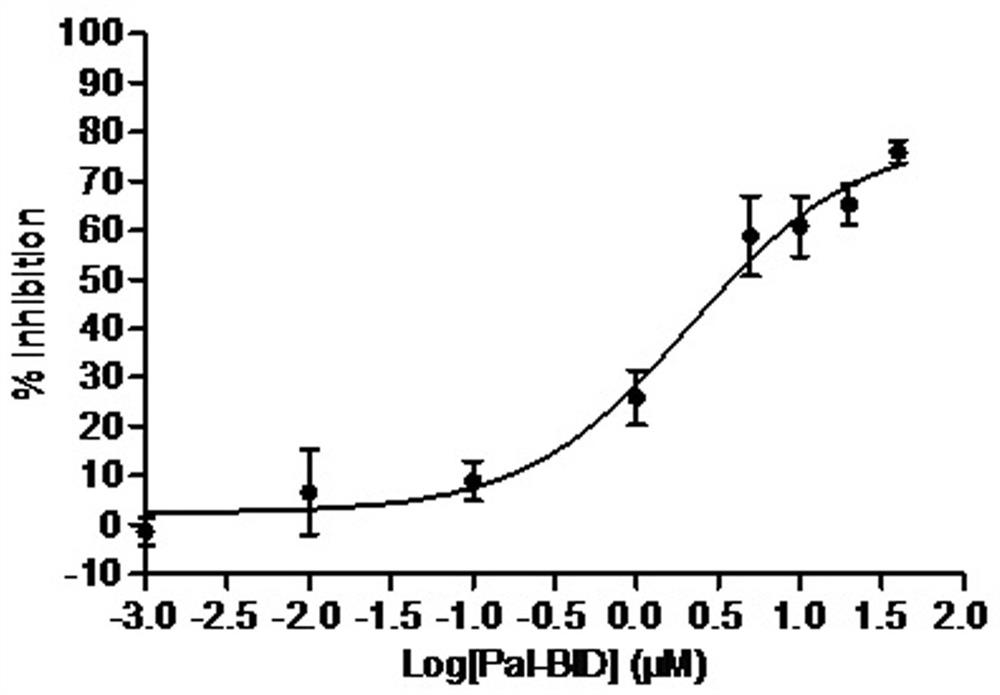
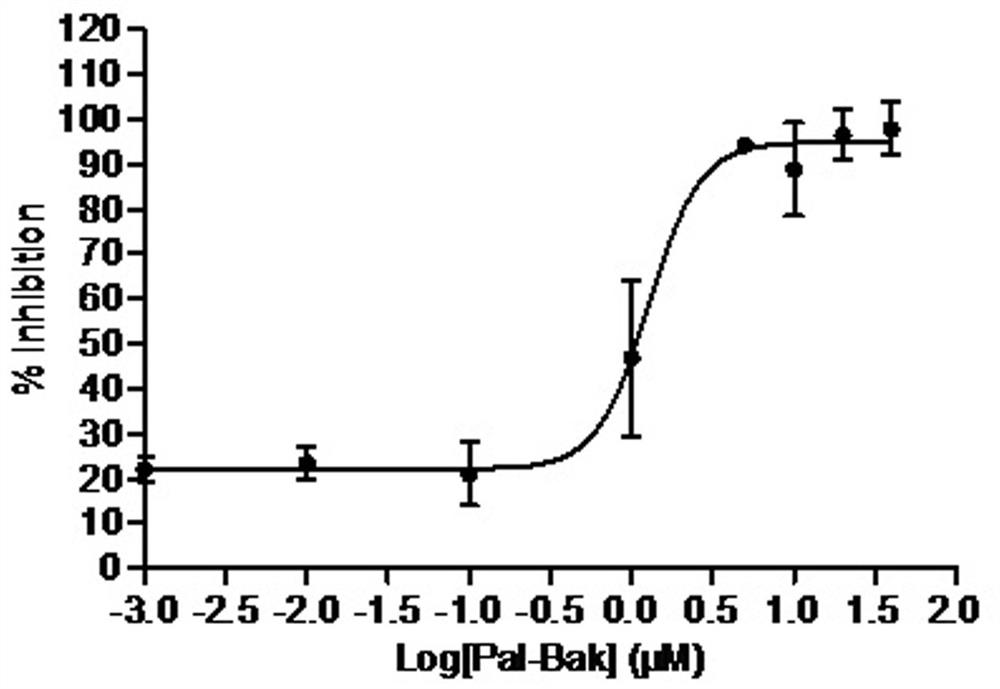
[0056] Example 3, Determination of protein tyrosine phospholipase 1B (PTP1B) inhibitory activity
[0057] In the present invention, MES buffer is adopted as the reaction system, human protein tyrosine phosphatase 1B (PTP1B) is utilized, disodium p-nitrophenylphosphate (pNPP) is used as the specific substrate, and sodium orthovanadate is selected as the positive drug , With DMSO as negative control, a screening model based on enzyme reaction rate of 96-well microplate as carrier was established, and PTP1B inhibitors were found by enzymatic methods.
[0058] The specific implementation method is: using MES buffer system (25mM, pH6.5), sequentially add 10μL pNPP (77mM), 86μL MES buffer solution, 4μL compound (2mM compound mother solution dissolved in DMSO), 100μL PTP1B solution ( 50 nM), the total reaction volume was 200 μL. 3 parallels in each group, with DMSO as the negative control and sodium orthovanadate (2mM) as the positive control, at 25°C, shake on the shaker for 1min, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com