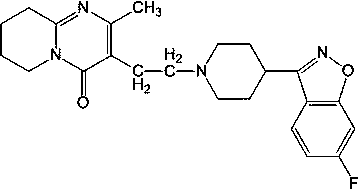
Risperidone oral solution and preparation method thereof
A technology for oral solution and risperidone, which is applied in the field of risperidone oral solution and its preparation, can solve problems such as slow clearance speed, and achieve the effects of rapid absorption, easy taking, and increased medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Prescription composition:
[0029] Preparation Process:
[0030] 1. Heat a certain amount of purified water to 75°C.
[0031] 2. Preparation of 1 mol / l sodium hydroxide solution: add the weighed sodium hydroxide to the quantitative purified water, stir and dissolve to make a 1 mol / l sodium hydroxide solution.
[0032] 3. Part of the purified water is transferred from step 1, a part of which is used to clean the container; the remaining part of the purified water is used to dissolve the material and make up the volume.
[0033] 4. Weigh quantitative purified water to fully dissolve the prescribed amount of benzoic acid, and record the experimental phenomenon.
[0034] 5. Weigh the recipe amounts of benzoic acid, tartaric acid and sucralose and add them to a beaker containing hot water in turn, keep heating at 70-80°C, and stir to completely dissolve the above materials, and judge the dissolution status by visual inspection. .
[0035] 6. After completely dissolving...
Embodiment 2
[0040] Prescription composition:
[0041] Preparation Process:
[0042] 1. Heat a certain amount of purified water to 75°C.
[0043] 2. Preparation of 1 mol / l sodium hydroxide solution: add the weighed sodium hydroxide to the quantitative purified water, stir and dissolve to make a 1 mol / l sodium hydroxide solution.
[0044] 3. Transfer part of purified water from step 1, part of which is used for cleaning the container and the remaining part of purified water is used for dissolving materials and making constant volume.
[0045] 4. Weigh the quantitative purified water to fully wet the prescription methylparaben and propylparaben, and record the experimental phenomenon.
[0046] 5. Take by weighing the recipe quantities of methylparaben, propylparaben, DL-tartaric acid, and sucralose and join them successively in the beaker containing hot water, keep heating at 70-80 °C simultaneously, and stir to make the above The material was completely dissolved, and the dissolution w...
Embodiment 3
[0052] Prescription composition:
[0053] Preparation Process:
[0054] 1. Heat a certain amount of purified water to 75°C.
[0055] 2. Preparation of 1 mol / l sodium hydroxide solution: add the weighed sodium hydroxide to the quantitative purified water, stir and dissolve to make a 1 mol / l sodium hydroxide solution.
[0056] 3. Transfer part of purified water from step 1, part of which is used for cleaning the container and the remaining part of purified water is used for dissolving materials and making constant volume.
[0057] 4. Weigh the quantitative purified water to fully wet the prescription amount of ethyl paraben, and record the experimental phenomenon.
[0058] 5. Weigh the recipe quantities of ethyl paraben, DL-tartaric acid, and sucralose and add them to a beaker containing hot water in turn, keep heating at 70-80°C, and stir to dissolve the above-mentioned materials completely. Dissolution was judged visually.
[0059] 6. After completely dissolving, the abo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com