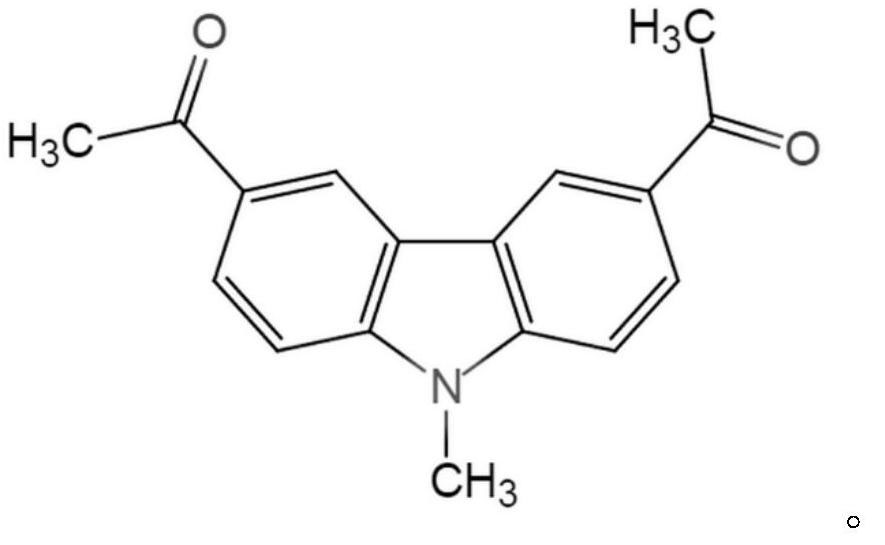
Use of 9-methyl-3,6-diacetylcarbazole for treating or preventing respiratory inflammatory diseases
A technology for diacetyl carbazole and inflammatory diseases, which is applied in the field of PI3Kγ-specific inhibitors, and can solve the problems of limited efficacy of drugs for respiratory inflammatory diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
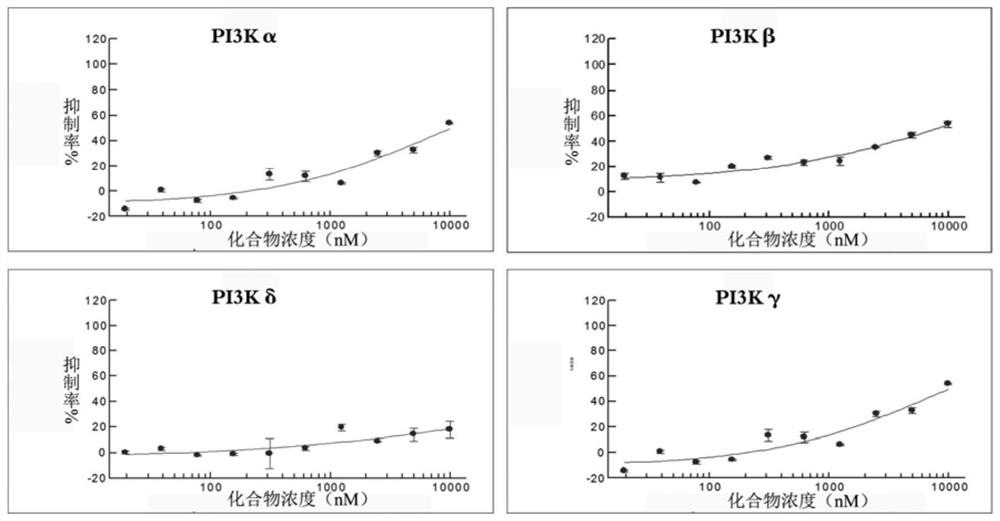
[0021] Embodiment 1: PI3K inhibition rate detected by in vitro enzyme experiment;
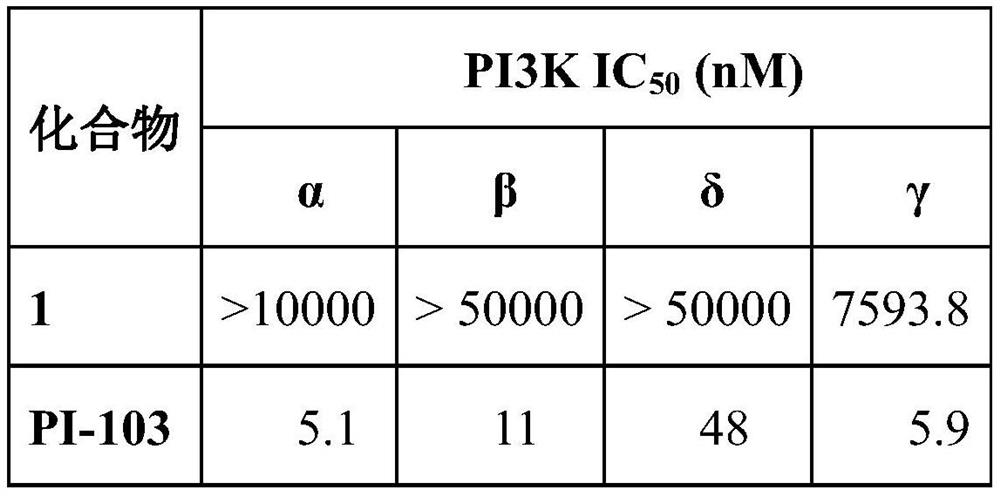
[0022] The inhibitory activity of the compound 9-methyl-3,6-diacetylcarbazole on PI3Kα, PI3Kβ, PI3Kδ, and PI3Kγ was detected using the ADP-Glo kit.
[0023] The broad-spectrum PI3K inhibitor PI-103 was used as a positive control.
[0024] PI3Kγ blank group: use a 96-well plate to set up 3 parallel experimental groups. Prepare 10 μl of PI3K reaction system, each well contains 2.5 μL of PI3Kγ protein, add 2.5 μL of reaction substrate PIP2 to start the reaction, incubate at room temperature for 1 hour, use 5 μL of MgCl 2 The reagent terminated the reaction, and after incubation for 2 hours, 10 μL of ADP-Glo detection reagent was added, and after incubation for 45 minutes, the fluorescence value of the reaction solution was detected by a microplate reader.
[0025] Use the same operation to prepare PI3Kα, PI3Kβ, PI3Kδ blank groups
[0026] PI3Kγ experimental group: use a 96-well plate and se...
Embodiment 2
[0033] Example 2: Detecting the inhibitory effect of compounds on the expression of pro-inflammatory cytokine TNFα;
[0034] ELISA kits were used to detect the content of TNFα in inflammatory cells.
[0035] (1) Take mouse mononuclear macrophages (RAW264.7 cells) in the logarithmic growth phase, inoculate 1 mL of 5000 cells per well on a 6-well plate, set up blank control group, LPS (lipopolysaccharide) group, and positive drug (LY294002)+LPS control group, 9-methyl-3,6-diacetylcarbazole+LPS group.
[0036] (2) The blank control group was not treated; the LPS group was treated with 1 μg / mL LPS; the LY294002 group was pretreated with 1 μM for 30 minutes and then added LPS (1 μg / mL) to treat the cells; 9-methyl-3,6-di In the acetylcarbazole group, three concentrations were set, and the cells were treated with 5, 10, and 20 μM for 30 minutes, and then LPS (1 μg / mL) was added to treat the cells. After culturing the cells for 6 hours, the cells were collected, centrifuged at 6000 ...
Embodiment 3
[0048] Embodiment 3: detecting the therapeutic effect of compound on asthmatic rat model;
[0049] (1) Take 30 SPF grade male SD rats, weighing about 200g, and divide the rats into 3 groups randomly: normal control group, OVA (ovalbumin)-induced asthma group and 9-methyl-3,6-di Acetylcarbazole-treated group. In the asthma group, 1 mL of OVA (concentration 0.8 g / L) was injected intraperitoneally on the 1st and 8th day, and on the 15th day, an air-compressed nebulizer was used to atomize and challenge with 1% OVA physiological saline solution in a closed plexiglass container. , 30 minutes each time, 3 times a week for 5 weeks. The normal control group was treated with normal saline instead of OVA, and the treatment method was the same. On the basis of the asthma group, the 9-methyl-3,6-diacetylcarbazole treatment group was administered intraperitoneally at a concentration of 20 mg / kg half an hour before the nebulization of 1% OVA normal saline solution.
[0050] (2) Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com