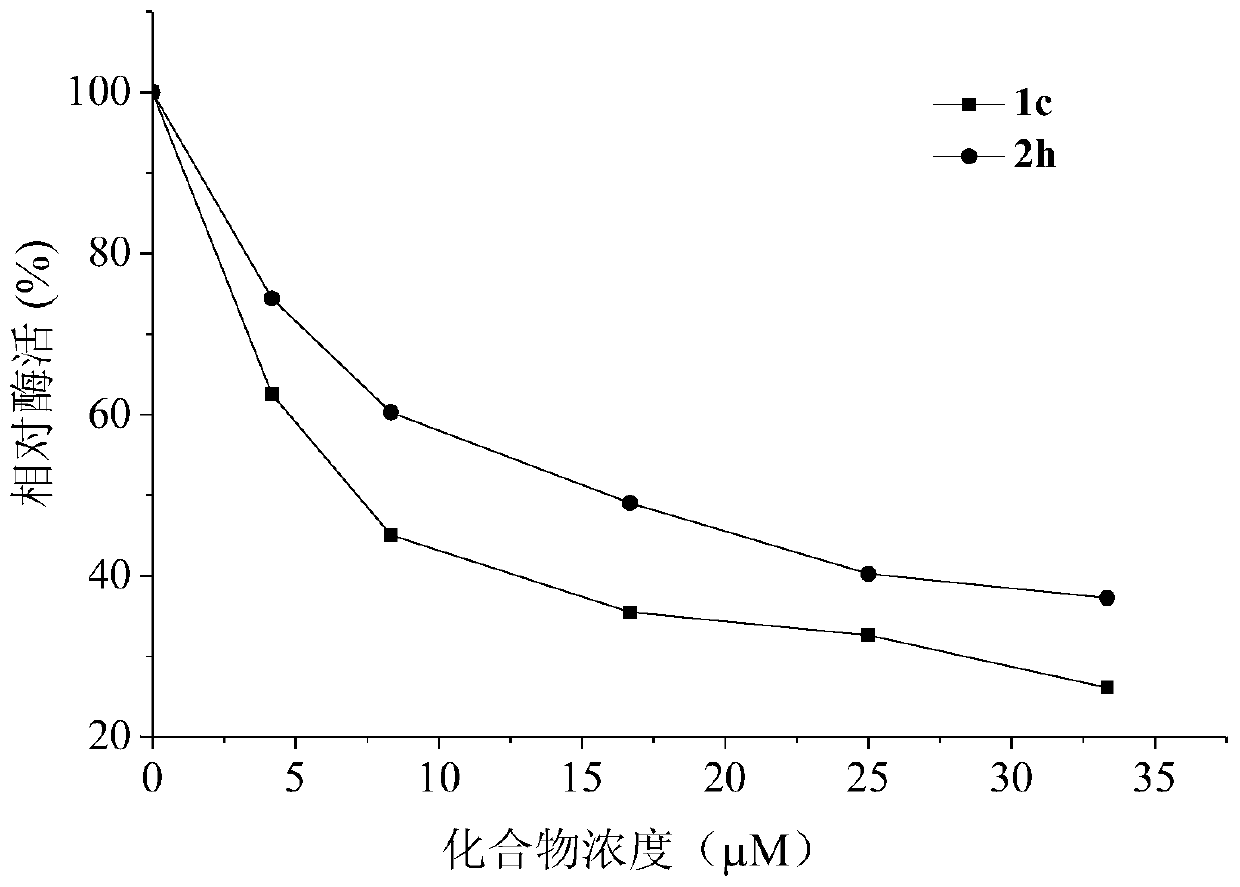
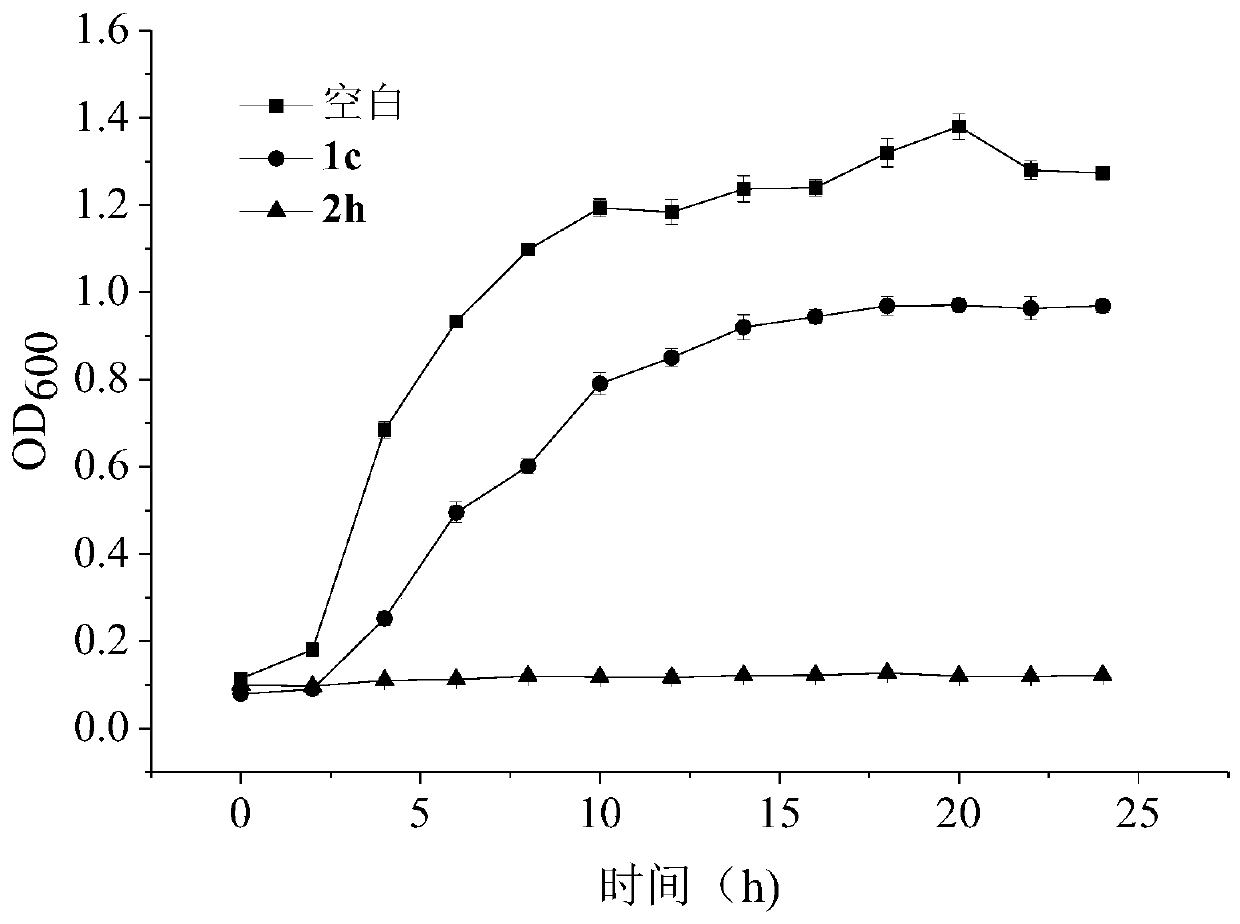
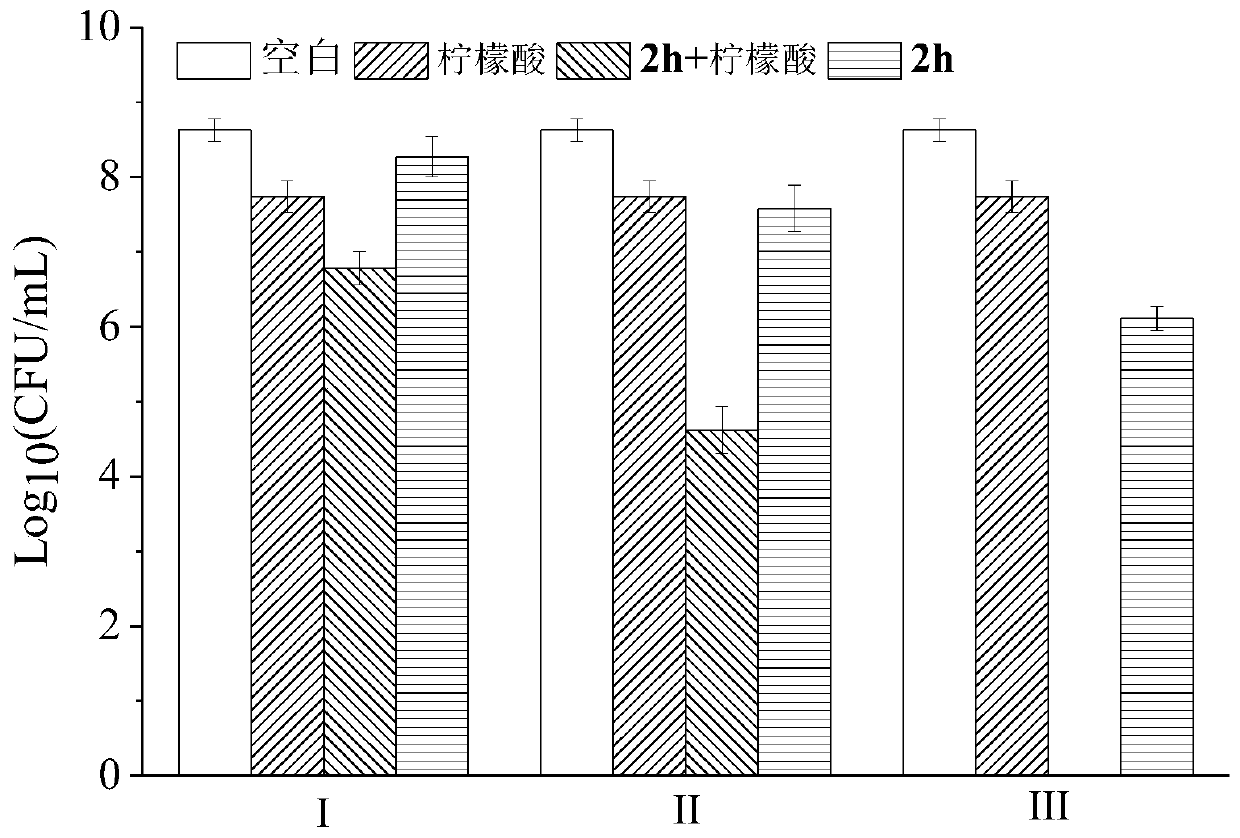
Stilbene analogous compounds and their preparation methods and uses
A compound, the technology of stilbenes, applied in the field of stilbenes similar compounds and their preparation and application, can solve the problems of high price, high toxicity, fresh-keeping application restrictions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0031] Embodiment 1-1, the preparation method of compound 1c, carry out the following steps successively:
[0032] Compound 4 was synthesized from kojic acid (3) according to the method reported in the literature (Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. Journal of Agricultural and Food Chemistry 2013, 61(27), 6597-6603) .
[0033] Compound 4: 5-benzyloxy-2-hydroxymethyl-pyran-4-one, yield 82.7%. 1 H NMR (400MHz, DMSO-d6, δppm) δ: 4.30(d, J=6.0Hz, 2H, CH 2 ),4.96(s,2H,CH 2 ), 5.67(t, J=6.0Hz, 1H, OH), 6.34(s, 1H, Py-H), 7.34-7.44(m, 5H, Ar-H), 8.17(s, 1H, Py-H) .ESI-HRMS: m / z, calculated value C 13 h 13 o 4 ([M+H] + ) 233.0808, measured value 233.0830.
[0034] A, the synthesis of compound 5
[0035] Weigh 30g (129.72mmol) of compound 4 (5-benzyloxy-2-hydroxymethyl-pyran-4-one) and dissolve it in 100mL of thionyl chloride, react at room temperature for 2h, after the reaction, suction filter, A pale yello...
Embodiment 1-2
[0050] Change the 3,5-dimethoxybenzaldehyde in Step C of Example 1-1 to 4-methoxybenzaldehyde and 3,4-dimethoxybenzaldehyde respectively, and the rest are the same as in Example 1-1 ; Thereby correspondingly obtaining compounds 1a, 1b.
[0051] The structural formula of compound 1a is:
[0052]
[0053] The structural formula of compound 1b is:
[0054]
Embodiment 2
[0055] Embodiment 2, the preparation method of compound 2h, carry out following steps successively:
[0056] Compound 4 was synthesized from kojic acid (3) according to the method reported in the literature (Design and synthesis of hydroxypyridinone-L-phenylalanine conjugates as potential tyrosinase inhibitors. Journal of Agricultural and Food Chemistry 2013, 61(27), 6597-6603) .
[0057] Compound 4: 5-benzyloxy-2-hydroxymethyl-pyran-4-one: yield 82.7%. 1 H NMR (400MHz, DMSO-d6, δppm) δ: 4.30(d, J=6.0Hz, 2H, CH 2 ),4.96(s,2H,CH 2 ), 5.67(t, J=6.0Hz, 1H, OH), 6.34(s, 1H, Py-H), 7.34-7.44(m, 5H, Ar-H), 8.17(s, 1H, Py-H) .ESI-HRMS: m / z, calculated value C 13 h 13 o 4 ([M+H] + ) 233.0808, measured value 233.0830.
[0058] A, the synthesis of compound 5
[0059] Weigh 30g (129.72mmol) of compound 4 (5-benzyloxy-2-hydroxymethyl-pyran-4-one) and dissolve it in 100mL of thionyl chloride, react at room temperature for 2h, and suction filter after the reaction to obtain a large...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com