High-efficiency preparation method for 2.3.6.7-tetramethyl anthracene and application thereof in preparation of triptycene and derivatives thereof
A tetramethylanthracene, high-efficiency technology, applied in the application field of preparing triptycene and its derivatives, can solve the problems of endangering human health, harsh preparation conditions, and affecting the rapid development of triptycene synthesis chemistry, and achieves high feasibility , The preparation technology is simple, and the effect of promoting rapid development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
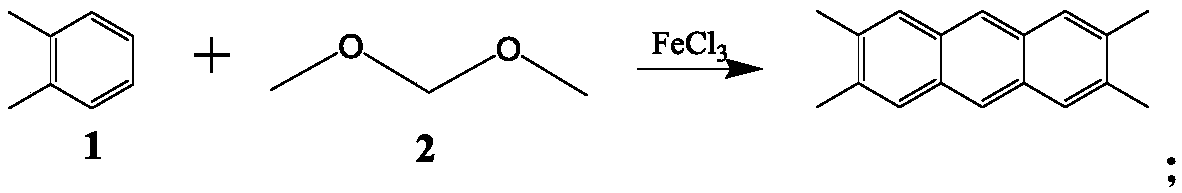
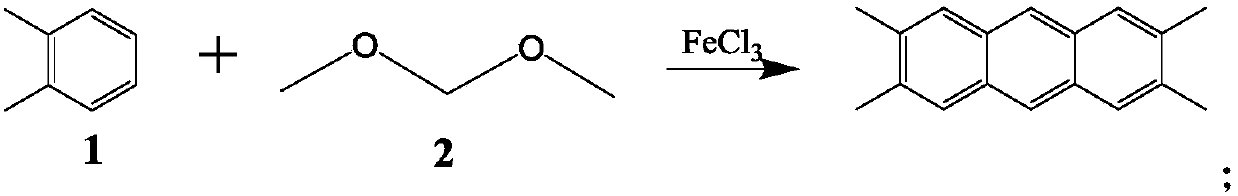
[0023] At room temperature, divide 8.2g (grams) of ferric trichloride into a 100ml three-necked flask equipped with 6ml (milliliters) of o-xylene and a mechanical stirrer, and slowly add 13ml (milliliters) of dimethoxymethane.
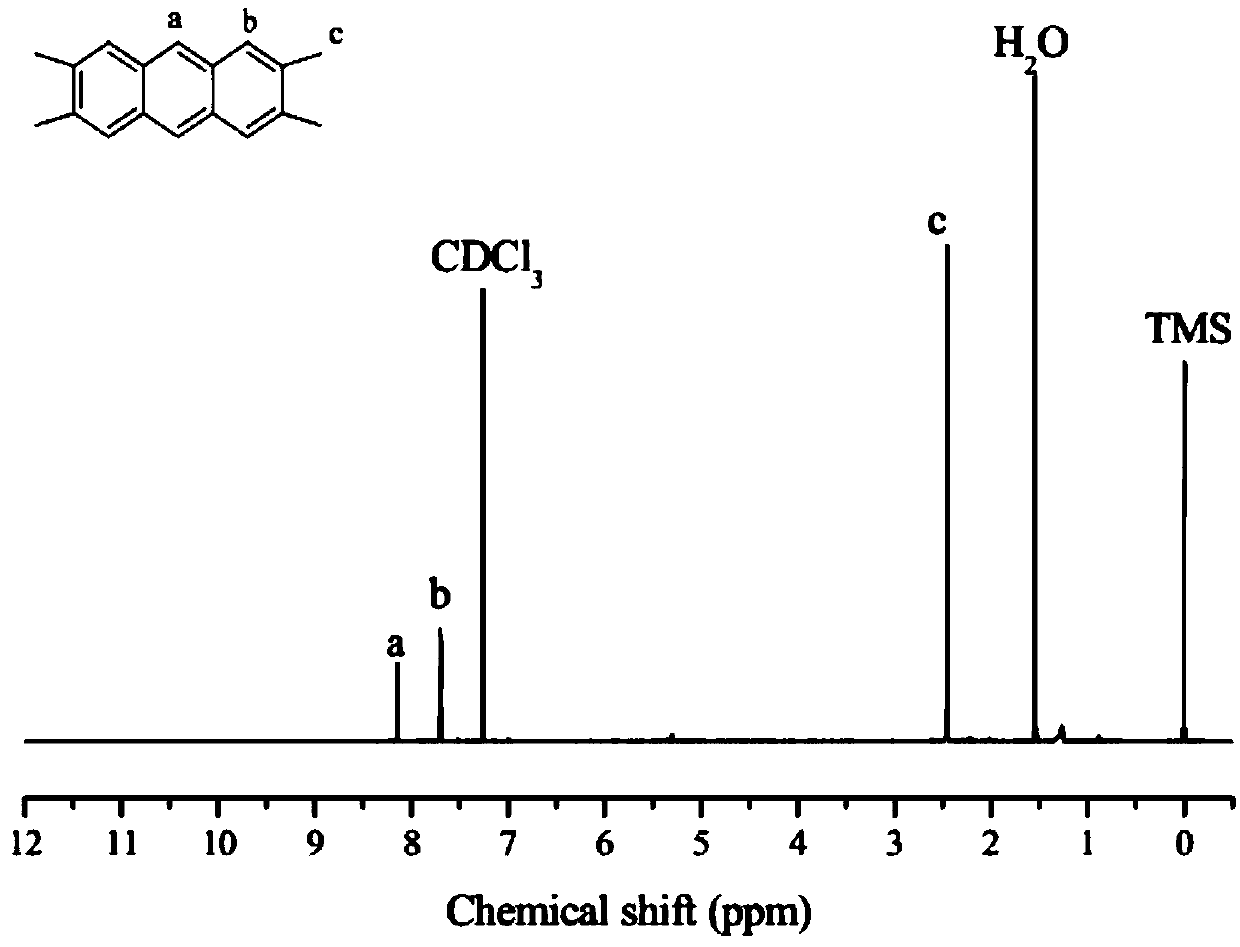
[0024] After the addition is complete, stir at room temperature for 1 hour (hour), and then react in a water bath at 70° C. for 4 hours. After the water bath is completed, drop the ice bath to below 5°C, pour about 40mL of 3mol / L sulfuric acid ice-water mixed solution, stir at room temperature for 30 minutes, pour out the upper layer containing salt, add 50mL of xylene, heat, stir and reflux for 0.5h, and cool to Suction filtration at room temperature, the filter cake was washed 3 times with xylene to obtain a black-green solid, which was dried under an infrared lamp in a fume hood, and extracted with xylene for 48 hours to obtain white needles 2,3,6, 4.7 g of 7-tetramethylanthracene crystals, yield 80.3%, melting point 299°C, purity 99.8%.
[0025] ...
Embodiment 2
[0029] At room temperature, divide 16.4g (grams) of ferric chloride into three additions into a 250ml three-necked flask equipped with 12ml (milliliters) of o-xylene and a mechanical stirrer, and slowly add 26ml (milliliters) of dimethoxymethane.
[0030] After the addition is complete, stir at room temperature for 1 hour (hour), and then react in a water bath at 70° C. for 4 hours. After the water bath is completed, drop the ice bath to below 5°C, pour about 80mL of 3mol / L sulfuric acid ice-water mixed solution, stir at room temperature for 30 minutes, pour out the upper layer containing salt, add 100mL of xylene, heat, stir and reflux for 0.5h, and cool to Suction filtration at room temperature, the filter cake was washed 3 times with xylene to obtain a black-green solid, which was dried under an infrared lamp in a fume hood, and extracted with xylene for 48 hours to obtain white needles 2,3,6, 9.5 g of 7-tetramethylanthracene crystals, yield 81.2%, melting point 299°C, pur...
Embodiment 3
[0032] At room temperature, divide 8.2g (grams) of ferric trichloride into a 100ml three-necked flask equipped with 6ml (milliliters) of o-xylene and a mechanical stirrer, and slowly add 13ml (milliliters) of dimethoxymethane.
[0033] After the addition is complete, stir at room temperature for 1 hour (hour), and then react in a water bath at 80° C. for 4 hours. After the water bath is completed, lower the ice bath to below 5°C, pour about 40mL of 3mol / L sulfuric acid ice-water mixed solution, stir at room temperature for 30 minutes, pour out the upper layer containing salt, add 50mL of xylene, heat, stir and reflux for 1h, and cool to room temperature , filtered with suction, and the filter cake was washed 3 times with xylene to obtain a black-green solid, which was dried under an infrared lamp in a fume hood and extracted with xylene for 48 hours to obtain white needles 2,3,6,7 - 4.9 g of tetramethylanthracene crystals, the yield is 83.8%, the melting point is 299° C., and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com