Method for preparing NK by using freeze-dried feeder layer cells
A technology of feeder cells and cells, which is applied in the field of biomedicine, can solve problems such as low amplification efficiency, increased safety risks, and adverse effects, and achieve the effects of high cell viability, high killing effect, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] According to the method of the present invention, the concrete construction K562 engineered cell steps are as follows:
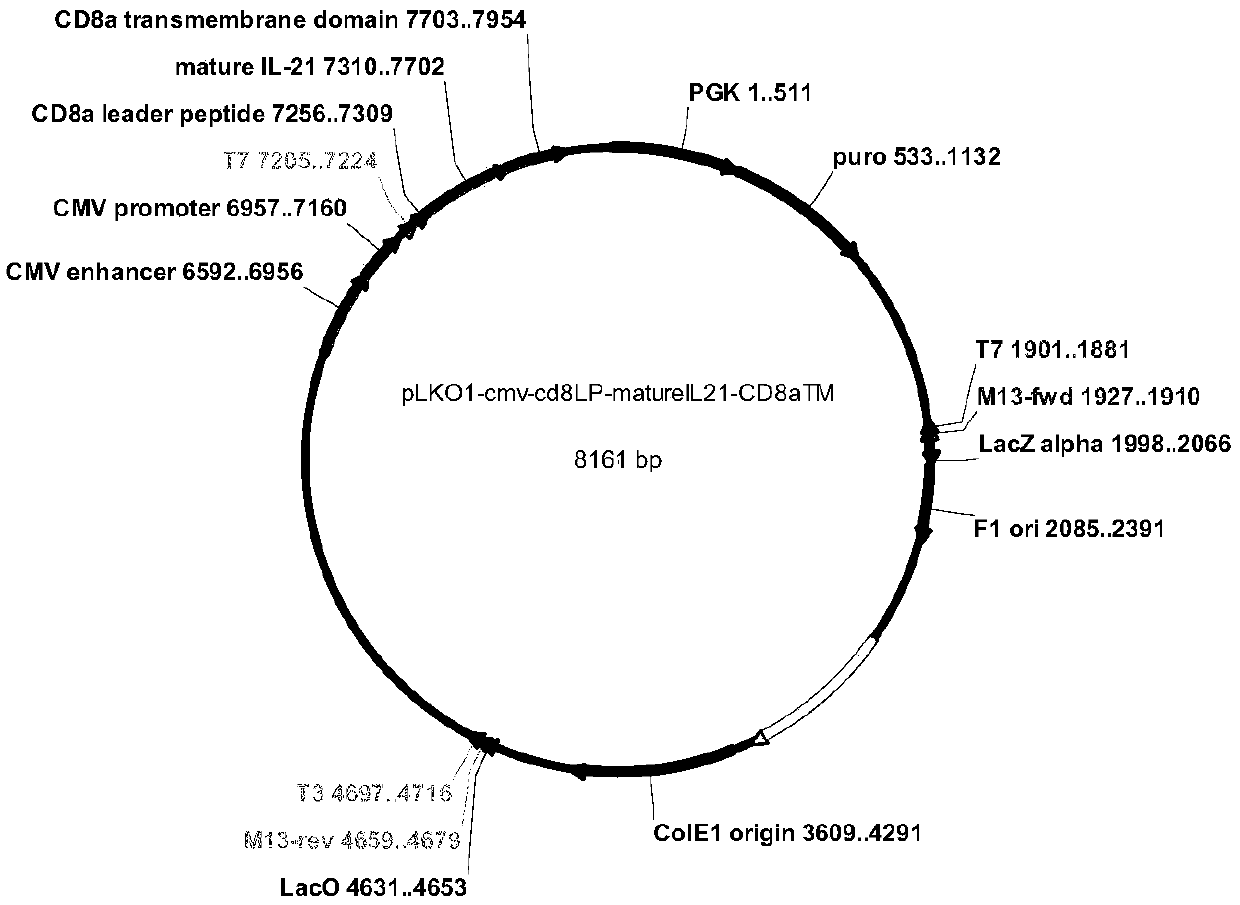
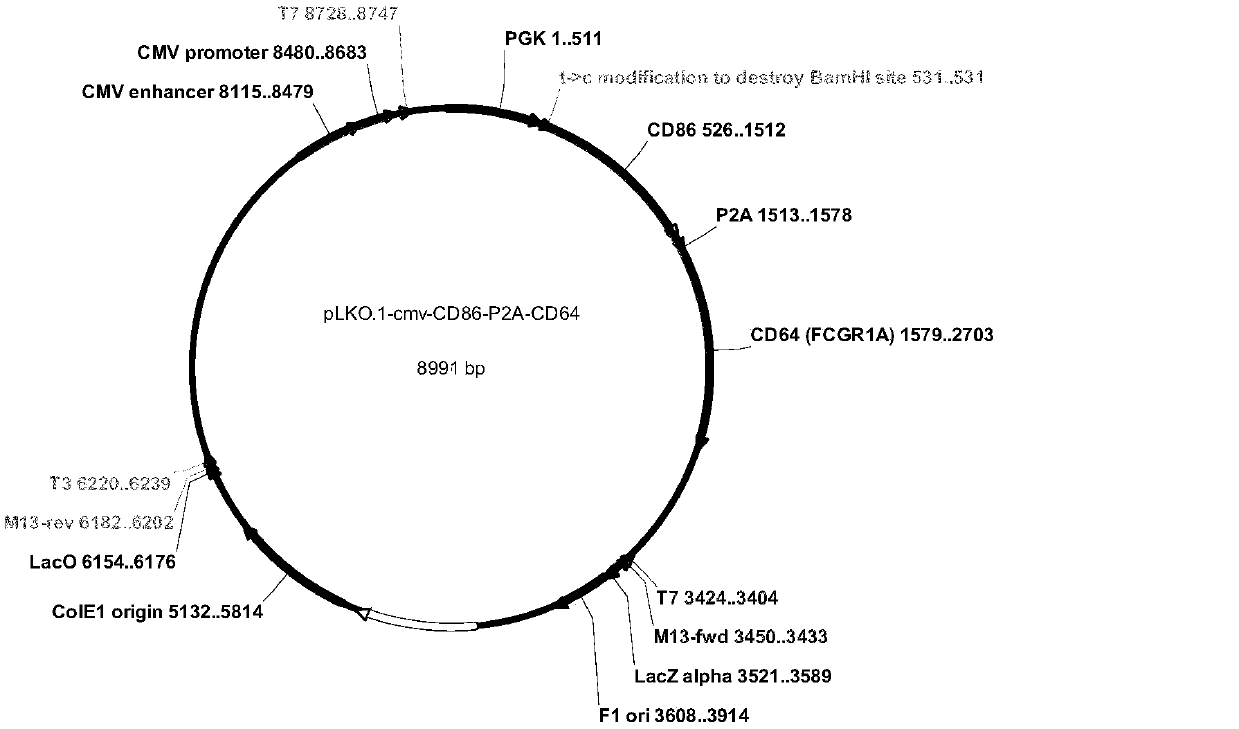
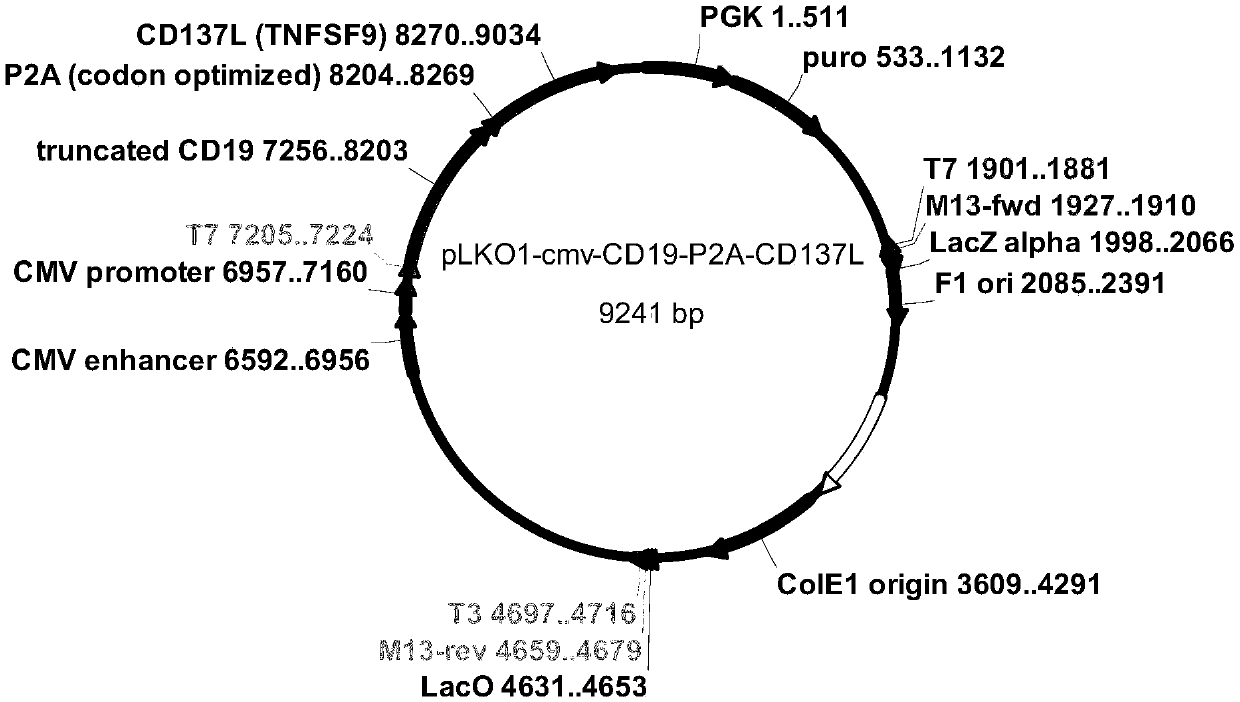
[0067] First, we synthesized five genes, corresponding to: CD64 (FccRI shown in sequence 1), CD86 (B7-2, shown in sequence 2), CD137L (4-1BBL, shown in sequence 3), truncated CD19 (shown in sequence 4) and membrane-anchored protein IL21 (shown in sequence 5); then subcloned to lentiviral vectors, we constructed the five proteins on three lentiviral vectors, the specific plasmid map is as follows figure 1 , 2 and 3 as shown;
[0068] First use the lentiviral vector pLKO-PGK-puro-CMV-CD8a (guide peptide, as shown in sequence 6)-IL-21-CD8a (transmembrane region, as shown in sequence 7) to construct IL21 on K562 cells, add 2ug / ml puromycin screening, and then flow cytometric detection and sorting, to obtain K562-IL21 cells with high expression of IL21; on this basis, the lentiviral vector pLKO-PGK-CD86-P2A-CD64 was used to construct CD86 and CD64 into K...
Embodiment 2
[0089] According to the method of the present invention, the concrete groping optimal trophoblast ratio step is as follows:
[0090] 1. Collect 30ml of peripheral blood.
[0091] 2. Add 15ml of Ficoll lymphocyte separation medium to two 50ml centrifuge tubes respectively (the lymphocyte separation medium is taken out in advance and placed at room temperature).
[0092] 3. Gently add 30ml of whole blood to two 15ml tubes of separation solution (without destroying the interface between the separation solution and blood).
[0093] 4. Put it into a horizontal centrifuge, balance it, centrifuge at 800g for 20min, and adjust the lifting speed to the lowest.
[0094] 5. After centrifugation, draw the upper serum to a new centrifuge tube for inactivation in a water bath at 56°C for 30 minutes, and place it on ice for 20 minutes.
[0095] 6. Carefully aspirate the buffy coat and transfer to a new 50ml centrifuge tube.
[0096] 7. Add more than 2 times the volume of normal saline, mi...
Embodiment 3
[0105] NK cell experiments under the optimal K562 engineered cell feeder layer ratio (blood from three different donors):
[0106] 1. Collect 10ml of peripheral blood from three donors respectively.
[0107] 2. Add 15ml of Ficoll lymphocyte separation medium to a 50ml centrifuge tube (the lymphocyte separation medium is taken out in advance and placed back to room temperature).
[0108] 3. Gently add 30ml of whole blood to 15ml of separation fluid (without destroying the interface between separation fluid and blood).
[0109] 4. Put it into a horizontal centrifuge, balance it, centrifuge at 800g for 20min, and adjust the lifting speed to the lowest.
[0110] 5. After centrifugation, draw the upper serum to a new centrifuge tube for inactivation in a water bath at 56°C for 30 minutes, and place it on ice for 20 minutes.
[0111] 6. Carefully aspirate the buffy coat and transfer to a new 50ml centrifuge tube.
[0112] 7. Add more than 2 times the volume of normal saline, mix ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com