Perovskite-solid solution composite photocatalyst used for photocatalytic water splitting to generate hydrogen
A solid solution and catalyst technology, which is applied in the field of perovskite-solid solution composite photocatalysts, can solve the problems of poor stability, low activity, and low quantum efficiency, and achieve the effects of improving stability, inhibiting recombination, and increasing output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
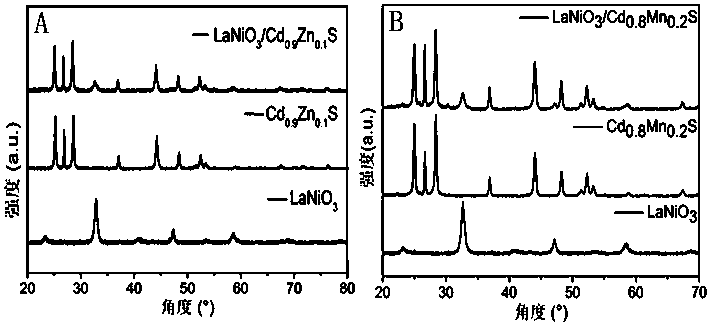
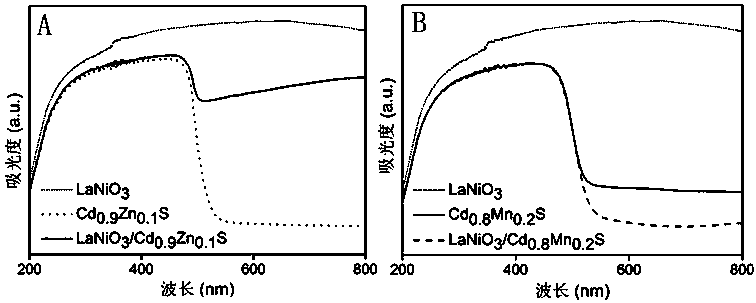
Image
Examples
Embodiment 1
[0022] Example 1 LaNiO 3 / Cd 0.9 Zn 0.1 Preparation of S composite catalyst
[0023] Dissolve 0.433g (1mmol) lanthanum nitrate and 0.290g (1mmol) nickel nitrate hexahydrate in 80mL deionized water, then add 20mL aqueous solution containing 0.3g (7.5mmol) sodium hydroxide, reflux at 60°C for 4h, the resulting precipitate After washing and drying, it was calcined at 650°C for 2 hours in an air atmosphere to obtain LaNiO 3 .
[0024] Take 4.797g (21mmol) of cadmium acetate and 0.439g (2.3mmol) of zinc acetate and dissolve them in a mixture of 30mL of ethylenediamine and 30mL of water, add 1.972g (26mmol) of thioacetamide, stir for 30min, and then transfer the solution to 100mL polytetrafluoroethylene stainless steel autoclave, and hydrothermal reaction at 200°C for 24h, finally washed with deionized water and ethanol, and dried at 60°C for 24h to obtain Cd 0.9 Zn 0.1 S solid solution.
[0025] LaNiO 3 and Cd 0.9 Zn 0.1 S was added to 20mL ethanol solution in proportion,...
Embodiment 2
[0026] Example 2 LaNiO 3 / Cd 0.8 mn 0.2 Preparation of S composite catalyst
[0027] Dissolve 0.433g (1mmol) lanthanum nitrate and 0.290g (1mmol) nickel nitrate hexahydrate in 80mL deionized water, then add 20mL aqueous solution containing 0.3g sodium hydroxide (7.5mmol), reflux at 60°C for 4h, the resulting precipitate After washing and drying, it was calcined at 650°C for 2 hours in an air atmosphere to obtain LaNiO 3 .
[0028] Take 4.264g (18mmol) of cadmium acetate and 0.7785g (4.5mmol) of manganese acetate and dissolve them in a mixture of 30mL of ethylenediamine and 30mL of water, add 1.737g (23mmol) of thioacetamide, stir for 30min, and then transfer the solution to 100mL polytetrafluoroethylene stainless steel autoclave, and hydrothermal reaction at 160°C for 24h, finally washed with deionized water and ethanol, and dried at 60°C for 24h to obtain Cd 0.8 mn 0.2 S solid solution.
[0029] LaNiO 3 and Cd 0.8 Zn 0.2 S was added to 20mL ethanol solution in propo...
Embodiment 3
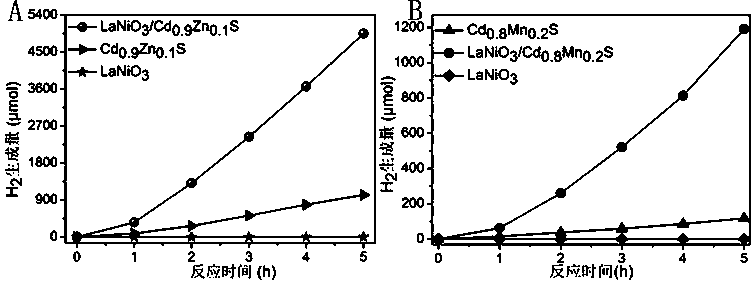
[0032] Example 3 LaNiO 3 / Cd x m (1-x) Evaluation of Hydrogen Production Activity of S Photolysis Water
[0033] Embodiment 1 and 2 gained LaNiO 3 / Cd x m (1-x) The evaluation experiment of the hydrogen production efficiency of S composite photocatalyst by photolysis of water was carried out on the Pofilai photolysis device, and Shimadzu 8A gas chromatograph was used to detect H 2 output. The experimental process is as follows: add 20 mg of the obtained composite photocatalyst to 100 mL of deionized water, and add 0.35 M Na 2 S / 0.25M Na 2 SO 3 As a hole sacrificial agent, use a xenon lamp as the light source and add a 420nm cut-off filter to carry out the photolysis reaction on the photowater splitting device. The system is vacuumed and the reaction temperature is 10°C. Illumination is carried out, and the H in the system is detected every 1h 2 content, the reaction was terminated after 5h.
[0034] image 3 For embodiment 1, 2 gained Cd x m (1-x) S. LaNiO 3 / Cd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com