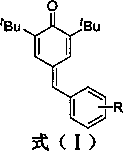
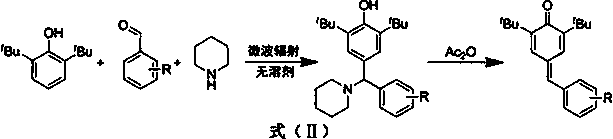
Synthesis of 4-phenylmethylene-2,6-di-tert-butyl-2,5-cyclohexadien-1-ketone through solvent-free microwave method
A technology of di-tert-butyl and benzylidene, applied in the field of organic synthetic chemistry, can solve the problems of large recrystallization loss, long operation time, and the need for solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Example 1 Preparation of 4-phenylmethylene-2,6-di-tert-butyl-2,5-cyclohexadien-1-one (A).
[0018] .
[0019] Weigh 2,6-di-tert-butylphenol (0.969 mmol), add benzaldehyde (1.163 mmol) into the reaction flask, raise the temperature to 120°C, add piperidine (0.969 mmol) dropwise, power 300 W, stir at constant temperature, and react for 30 Minutes; maintain temperature and power, add acetic anhydride (0.969 mmol), and stir for 3 minutes. After cooling, add water to the reaction system, extract the mixture with ethyl acetate and separate the layers, dry the organic phase with anhydrous sodium sulfate, and concentrate to obtain the crude product. The resulting crude product is subjected to column chromatography with an eluent whose volume ratio is ethyl acetate:petroleum ether=1:400 to obtain the prepared 4-phenylmethylene-2,6-di-tert-butyl-2,5 -Cyclohexadiene-1-one ( A ) pure product, yield 70.18%. 1 H NMR (400 MHz, CDCl 3 ) δ 7.45 (d, J = 2.2 Hz,1H), 7.37 (d, J =...
example 2
[0020] Example 2 Preparation of 4-(2-methoxyphenylmethylene)-2,6-di-tert-butyl-2,5-cyclohexadien-1-one ( B ).
[0021] .
[0022] Weigh 2,6-di-tert-butylphenol (0.969 mmol), o-methoxybenzaldehyde (1.163 mmol), and piperidine (0.969 mmol) into the reaction flask, heat up to 120°C, power 300 W, and stir at constant temperature. React for 30 minutes; maintain the temperature and power, add acetic anhydride (0.969 mmol), and stir for 3 minutes. After cooling, add water to the reaction system, extract the mixture with ethyl acetate and separate the layers, dry the organic phase with anhydrous sodium sulfate, and concentrate to obtain the crude product. The resulting crude product is subjected to column chromatography with an eluent whose volume ratio is ethyl acetate:petroleum ether=1:250 to obtain the prepared 4-(2-bromophenylmethylene)-2,6-di-tert-butyl The yield of 2,5-cyclohexadien-1-one ( B ) was 74.92%. 1 H NMR (600 MHz, CDCl 3 ) δ 7.46 (d, J =2.1 Hz, 1H), 7.43 - 7.32...
example 3
[0023] Example 3 Preparation of 4-(4-methoxyphenylmethylene)-2,6-di-tert-butyl-2,5-cyclohexadien-1-one (C).
[0024] .
[0025] Weigh 2,6-di-tert-butylphenol (0.969 mmol), add p-methoxybenzaldehyde (1.163 mmol) into the reaction flask, raise the temperature to 120°C, add piperidine (0.969 mmol) dropwise, power 300 W, constant temperature Stir and react for 30 minutes; maintain the temperature and power, add acetic anhydride (0.969 mmol) and stir for 3 minutes. After cooling, add water to the reaction system, extract the mixture with ethyl acetate and separate the layers, dry the organic phase with anhydrous sodium sulfate, and concentrate to obtain the crude product. The resulting crude product is subjected to column chromatography with an eluent whose volume ratio is ethyl acetate:petroleum ether=1:150 to obtain the prepared 4-(4-methoxyphenylmethylene)-2,6-bis tert-butyl-2,5-cyclohexadien-1-one ( C ) pure product, yield 58.33%. 1 H NMR (600 MHz, CDCl 3 ) δ 7.5 -7.6 (d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com