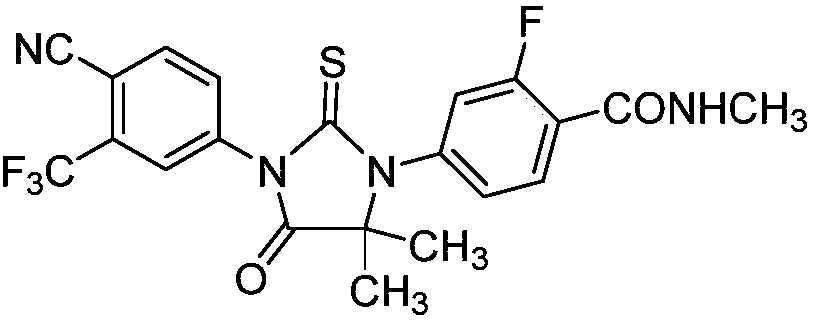
Preparation method of enzalutamide of formula (VIII)
A kind of enzalutamide, generative technology, applied in the field of chemical drug synthesis, can solve the problems of large environmental pollution, unstable intermediates, high cost, etc., to avoid the production of isothiocyanate intermediates, reagents are cheap and easy to obtain , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Under nitrogen protection, compound I (50.0 g, 0.267 mol) and compound N-tert-butoxycarbonyl-2-methylalanine (60.1 g, 0.295 mol) were added to a 1 L three-necked flask, and 150 mL of anhydrous THF was added at room temperature Stir until the solid is completely dissolved, add DIPEA (38.2g, 0.295mol) and control the reaction temperature at 10-30°C, then add 50mL of anhydrous THF, stir at room temperature for 10-15min, add CDI (52.3g, 0.322mol) in batches, The internal temperature was raised to 50-60°C and stirred for 30 minutes. Add DBU (61.3 g, 0.403 mol) and react at 55-65° C. for 3-4 h to stop the reaction. Add 150 mL of citric acid aqueous solution at 60°C and stir for 30 min, separate the liquid, concentrate the organic phase, and replace the solvent with ethanol, recrystallize with ethanol, filter with suction, and dry to obtain 45.8 g of white solid, with a yield of 49.5%. 1 H-NMR (400M, DMSO-d6):
[0039] δ10.292(s,1H),8.372(s,1H),8.176-8.154(d,1H,J=8.8Hz),8.07...
Embodiment 2
[0041]Under the protection of nitrogen, the amino protecting group PG is tert-butoxycarbonyl compound III (100g, 0.269mol) into a 500mL three-neck flask, add 300mL of 4M HCl isopropanol solution, and heat to 55-65°C for 2h. Stop the reaction, concentrate to 100mL, add 100mL of isopropanol, concentrate again to 100mL, add 300mL of ammonia solution in an ice-water bath, stir for 30min, filter with suction, wash the filter cake with 100mL of water, filter with suction to dryness, and dry at 60°C to obtain a white color Solid compound (IV) 68.1g, yield 93.33%. 1 H-NMR (400M, DMSO-d6):
[0042] δ8.463-8.458(d,1H,J=2.0Hz),8.216-8.189(dd,1H,J=8.4Hz,2.0Hz),8.097-8.071(dt,1H,J=8.4Hz,1.0Hz), 5.062(brs,3H),1.303(s,6H)
Embodiment 3
[0044] Under the protection of nitrogen, put the amino protecting group PG as tert-butoxycarbonyl compound III (100g, 0.269mol) into a 500mL three-neck flask, add 300mL of 4M HCl methanol solution, heat to 50-60°C for 1h, and stop the reaction , concentrated to 100mL, added 100mL of methanol, concentrated again to 100mL, added 300mL of ammonia solution in an ice-water bath, and stirred for 30min, filtered with suction, washed the filter cake with 100mL of water, filtered with suction to dryness, and dried at 55°C to obtain a white solid compound (IV )67.0g, yield 91.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com