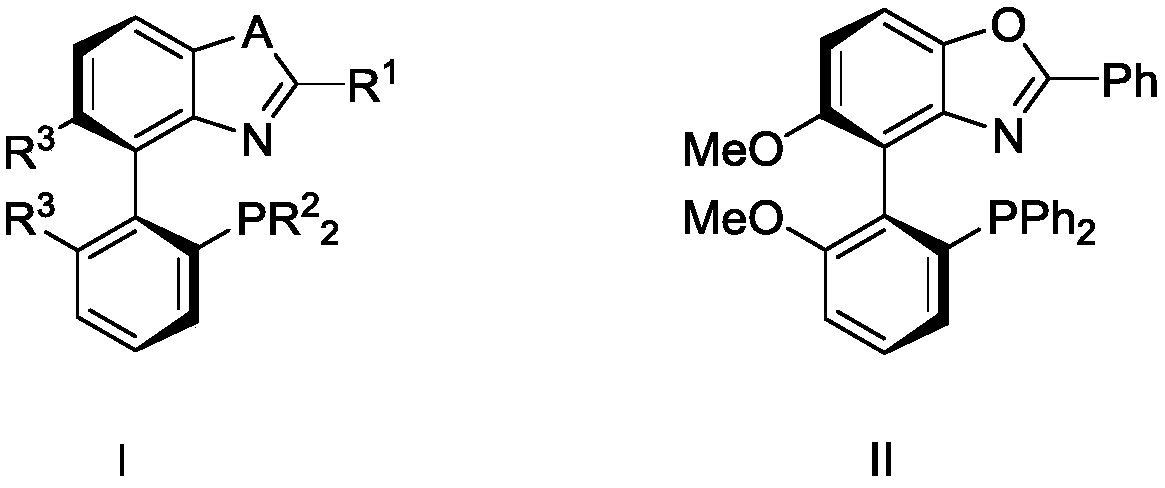
Chiral nitrogen-phosphorus ligand and preparation method thereof, and method for splitting racemic menthol
A technology of menthol and nitrogen and phosphorus is applied in the field of obtaining chiral menthol through kinetic resolution, can solve the problems of low yield, harsh conditions, low optical purity and the like, achieves low catalyst cost, simple equipment requirements, The effect of high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment i
[0057]
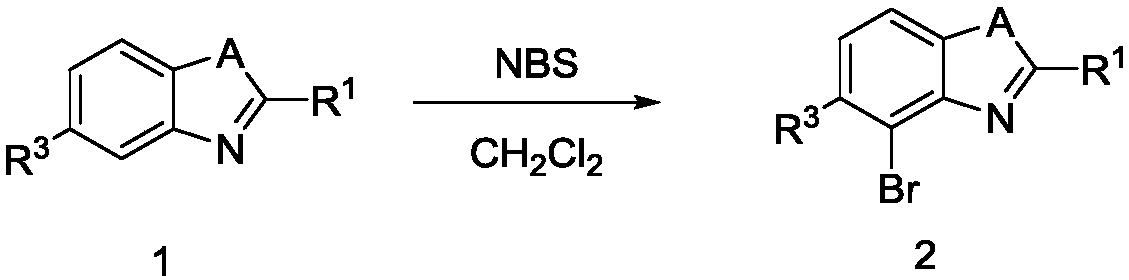
[0058] N-bromosuccinimide NBS (34.0 mmol) was added in portions to a solution of compound 1a (30.9 mmol) in dichloromethane (300 mL) at room temperature. After stirring at 30° C. for 2 hours, dichloromethane was removed from the reaction solution under reduced pressure, and the residue was further purified by distillation under reduced pressure to obtain compound 2a (96% yield).
[0059]
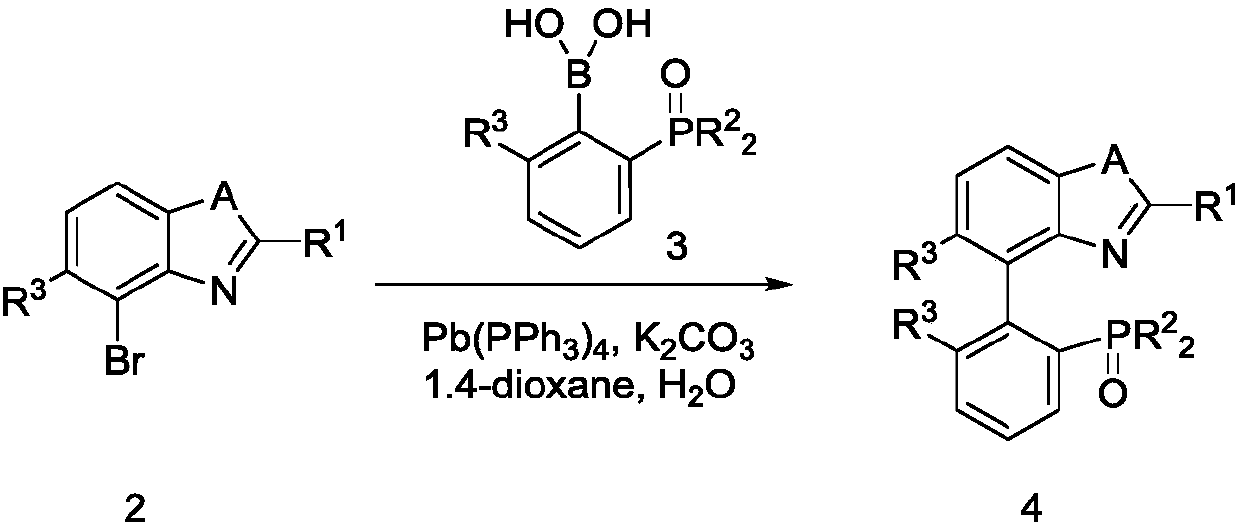
[0060] At room temperature, add compound 2 (34.0mmol), substituted phenylboronic acid 3 (34.0mmol), tetrakis (triphenylphosphine) palladium (3.4mmol), potassium carbonate (34.0mmol), 1,4-diox Hexacyclic (200 mL) and water (100 mL). Heating to reflux for 3 hours, after cooling, the reaction solution was filtered with diatomaceous earth, the filtrate was back-extracted with dichloromethane, the organic phase was concentrated under reduced pressure, and the raffinate was recrystallized with dichloromethane / n-hexane to obtain compound 4 (yield 98%).
[0061]
[0062] Add comp...
Embodiment 1
[0096] In a 2L stainless steel (316L material) reaction kettle, add 1.34 grams of (1,5-cyclooctadiene) iridium (I) dichloride dimer, 156.27 grams of tetrahydrofuran, 1.932 grams 201.6 grams of allyl acetate and 156.27 grams of racemic menthol; keep the stirring speed at 800 rpm, and react at 25°C for 2 hours. After the reaction solution was cooled to room temperature, the reaction solution was taken out, filtered with a Bourg-style funnel, and the solid was filtered out, and 40 wt% aqueous sodium hydroxide solution and 156.27 g of tetrahydrofuran were added to the solid with the same mass as the solid, stirred for 0.5 h, and left to stand For a period of time, the reaction solution was subjected to rotary evaporation, and finally vacuum distillation to obtain pure L-menthol, and the filtrate filtered by the Buchner funnel was poured out and spin-dried, and vacuum distillation was obtained to obtain pure D-menthol. Sampling Analysis, the results are shown in Table 1.
Embodiment 2
[0098] In a 2L stainless steel (316L material) reaction kettle, add 67.17 grams of (1,5-cyclooctadiene) iridium (I) dichloride dimer, 156.27 grams of tetrahydrofuran, 96.636 grams 201.6 grams of allyl acetate and 156.27 grams of racemic menthol; keep the stirring speed at 800 rpm, and react at 25°C for 2 hours. After the reaction solution was cooled to room temperature, the reaction solution was taken out, filtered with a Bourg-style funnel, and the lower layer of solid was removed, and 40 wt% aqueous sodium hydroxide solution and 156.27 g of tetrahydrofuran were added to the solid, stirred for 0.5 h, and then allowed to stand for a period of time. time, the reaction solution was subjected to rotary evaporation, and finally vacuum distillation to obtain pure L-menthol. The upper layer of the Buchner funnel was poured out and spin-dried, and finally vacuum distillation was obtained to obtain pure D-menthol. Sampling and analysis , the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com