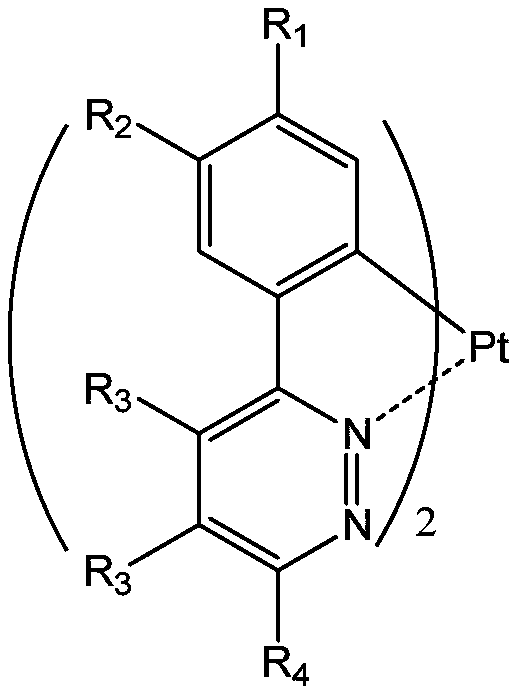
Double-ring metal platinum (II) coordination compound taking pyridazine derivative as ligand as well as preparation method and application of coordination compound
A technology of pyridazine derivatives and complexes, applied in the field of bicyclic metal platinum complexes and their preparation, can solve the problems of unsatisfactory luminescent properties of products, cumbersome synthetic routes, expensive raw materials, etc., to reduce the probability of redox and prevent molecular aggregation , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
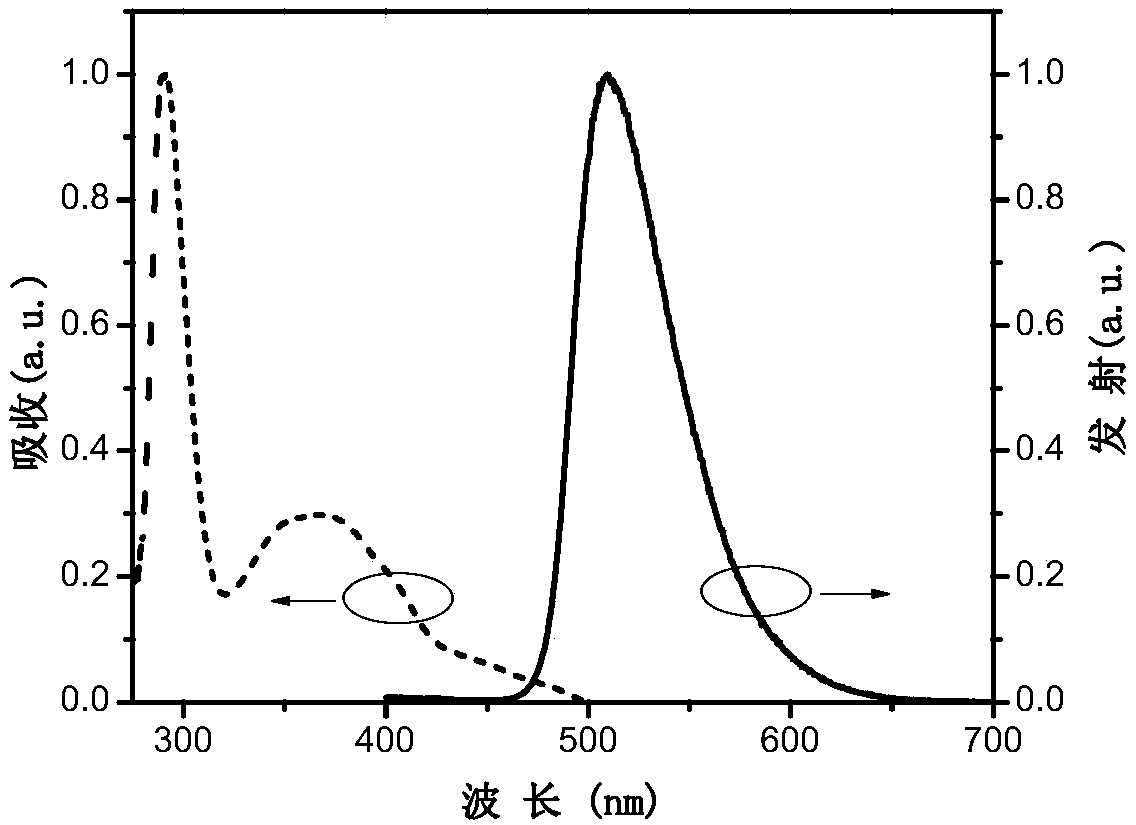
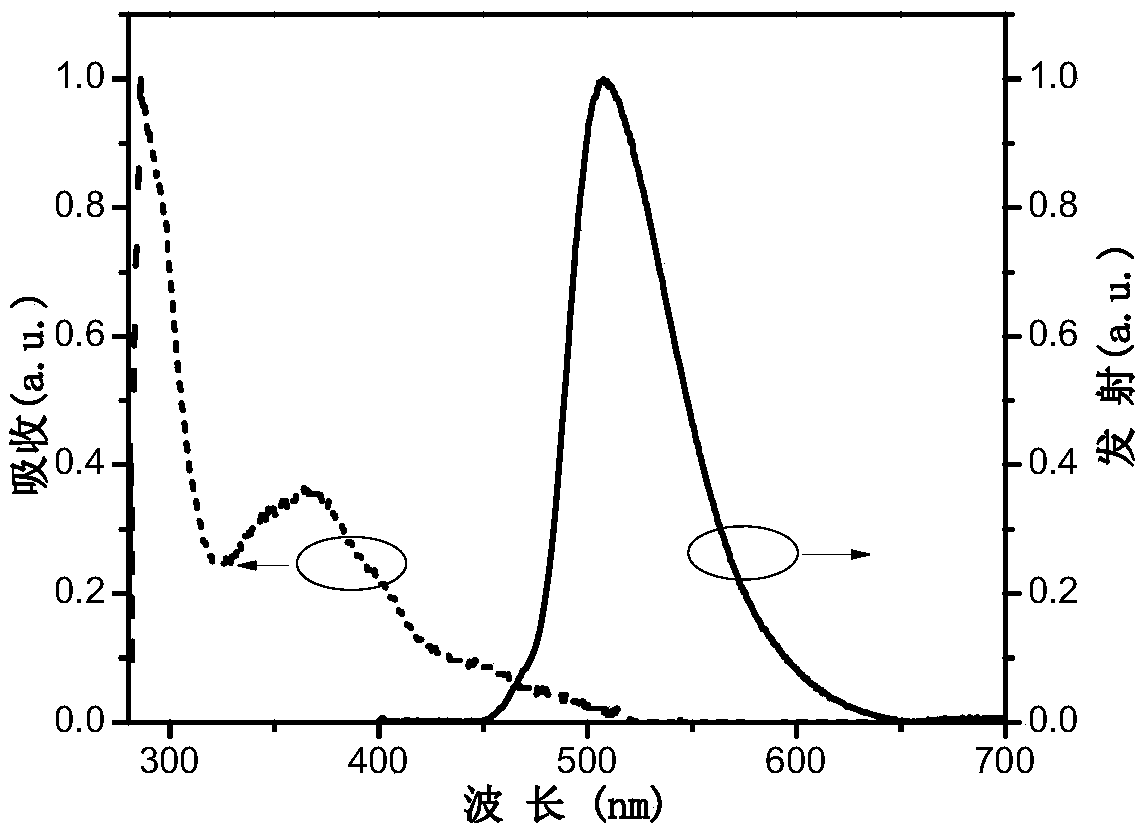
Image
Examples
Embodiment 1
[0048] 2.3g (10mmol) 3,6-dichloro-4,5-cyclohexyl (1',4') pyridazine, 3.8g (20mmol) m-trifluoromethylbenzeneboronic acid and 1.4g (10mmol) potassium carbonate Add to a 250mL round bottom flask, add 0.2g (0.3mmol) PdCl 2 (dppf), then pour 30mL tetrahydrofuran and 15mL water, N 2 Protection, reflux at 100°C for 12h. Cool, spin dry THF, extract with dichloromethane to obtain the lower organic phase, spin dry, and use petroleum ether: ethyl acetate = 1:1 eluent silica gel column chromatography to obtain 4.1g 3,6-bis( Trifluoromethylphenyl)-4,5-cyclohex(1',4')pyridazine as a white solid in 91% yield. The NMR spectrum of the product is: 1 HNMR (400MHz, CDCl 3 )δ8.00(s, 2H), 7.92(d, J=7.6Hz, 2H), 7.82(d, J=7.8Hz, 2H), 7.72(t, J=7.7Hz, 2H), 3.43(s, 2H), 1.98 (d, J = 7.9 Hz, 4H), 1.52 (d, J = 7.5 Hz, 4H). 19 F NMR (CDCl 3 , 367MHz) δ-62.86 (s, 6F). The mass spectrum of product is: MS ((+)-ESI): m / z=449.67 (calcd.449.14forC 24 h 19 f 6 N 2 , [M+H + ]).
[0049] 1.8g (4mmol)...
Embodiment 2
[0052] Add 10g (68mmol) of 3,6-dichloropyridazine, 12.8g (68mmol) of m-trifluoromethylbenzeneboronic acid and 9.38g (68mmol) of potassium carbonate into a 500mL round bottom flask, and add [1,1 , -bis(diphenylphosphino)ferrocene]palladium dichloride (PdCl 2 (dppf)) 1.1g (1.6mmol), then pour 45mL tetrahydrofuran and 30mL water, N 2 Protection, reflux for 12h. Cooled, spin-dried THF, extracted with dichloromethane solution, spin-dried the organic phase, and separated by silica gel column chromatography with petroleum ether:ethyl acetate=3:1 eluent to obtain 10.2g 3-(3-trifluoromethyl Phenyl)-6-chloropyridazine as a white solid, yield 58%. The NMR and MS data of the product are: 1 H NMR (400MHz, CDCl 3 )δ8.31(s, 1H), 8.26(d, J=7.8Hz, 1H), 7.88(d, J=9.0Hz, 1H), 7.78(d, J=7.8Hz, 1H), 7.67(t, J=7.8Hz, 1H), 7.62(d, J=9.0Hz, 1H); 19 F NMR (376MHz, CDCl 3 )δ-62.66 (s, 3F).MS ((+)-ESI): m / z=259.02 (calcd.259.03forC 11 h 7 CIF 3 N 2 , [M+H + ]).
[0053] Add 5 g (19 mmol) 3-...
Embodiment 3
[0057] 3-(3-trifluoromethylphenyl)-6-chloropyridazine 1.3g (5mmol), 2,4,6-trimethylphenol 0.8g (6mmol) were placed in a 120mL reaction flask, and the reaction Add potassium carbonate 1.4g (10mmol) in the bottle, with N,N-dimethylformamide 50mL as solvent, pass into N 2 Sealing protection, the reaction was refluxed for 12 hours, the reaction was completed and cooled to room temperature, the organic phase was extracted with ethyl acetate, and the organic phase was back-extracted with water, and the organic phase was spin-dried with petroleum ether and ethyl acetate at a ratio of 5:1. After separation by column chromatography, 1.4 g of white solid 3-(3-trifluoromethyl)-6-(2,4,6-trimethylphenoxy)pyridazine was obtained with a yield of 76%. The NMR and MS data of the product are: 1 H NMR (400MHz, CDCl 3 )δ8.34(s, 1H), 8.23(d, J=8Hz, 1H), 7.93(d, J=9.2Hz, 1H), 7.72(d, J=8.0Hz, 1H), 7.63(t, J =8.0Hz, 1H), 7.27(t, J=6.0Hz, 1H), 7.27(s, 2H), 2.32(s, 3H), 2.15(s, 6H); 19 F NMR (376M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermogravity temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com