Preparation method of s-isomer indoxacarb
A technology of indoxacarb and a system is applied in the field of preparation of S-indoxacarb, which can solve the problems of unrecyclable catalyst, high production cost, low catalyst efficiency, etc., and achieves benefits of industrialization, improved reaction speed, and enhanced selectivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
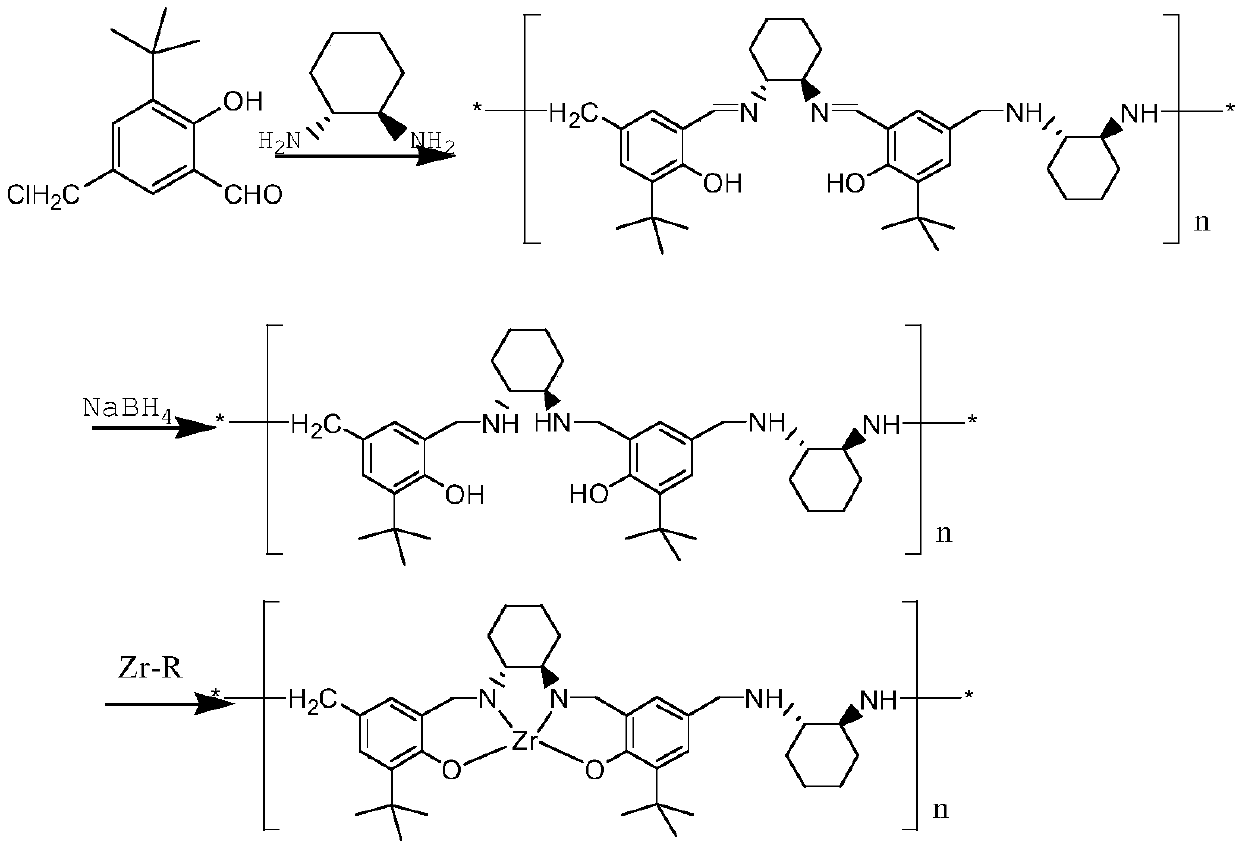
[0037] Embodiment 1 A chiral catalyst containing metal zirconium and a method for preparing S body indoxacarb using the catalyst, the preparation method is as follows:
[0038] (1) Add 22.65g (0.1mol) 3-tert-butyl-5-chloromethyl salicylaldehyde to 100mL clean anhydrous reaction flask, then add 50mL methanol, then weigh 11.4g (0.1mol) cyclohexanedi Amine, slowly added dropwise at 20-25°C.
[0039] (2) After the dropwise addition, the temperature was raised to 65-70°C for reflux reaction. After the reaction, the temperature was lowered to 10°C, and the ligand polymer was obtained by filtration, dried and weighed.
[0040] (3) 2000 mL of a clean anhydrous reaction bottle was put into the ligand polymer in step (2), then 50 mL of toluene and 3.78 g (0.1 mol) of sodium borohydride were added, and the temperature was raised to reflux for reaction.
[0041] (4) Add 24.35 g (0.05 mol) of zirconium acetylacetonate to the above reaction system, fully soak and heat up to 80° C., and kee...
Embodiment 2
[0045] Embodiment 2 A chiral catalyst containing metal zirconium and a method for preparing S body indoxacarb using the catalyst, the preparation method is as follows:
[0046] (1) Add 22.65g (0.1mol) 3-tert-butyl-5-chloromethyl salicylaldehyde to 100mL clean anhydrous reaction flask, then add 50mL methanol, then weigh 17.1g (0.15mol) cyclohexanedi Amine, slowly added dropwise at 20-25°C.
[0047] (2) After the dropwise addition, the temperature was raised to 65-70°C for reflux reaction. After the reaction, the temperature was lowered to 10°C, and the ligand polymer was obtained by filtration, dried and weighed.
[0048] (3) 2000 mL of a clean anhydrous reaction bottle was put into the ligand polymer in step (2), then 50 mL of toluene and 5.4 g (0.1 mol) of potassium borohydride were added, and the temperature was raised to reflux for reaction.
[0049] (4) Add 24.35 g (0.05 mol) zirconium acetylacetonate to the above reaction system, fully swell and heat up to 80° C., heat p...
Embodiment 3
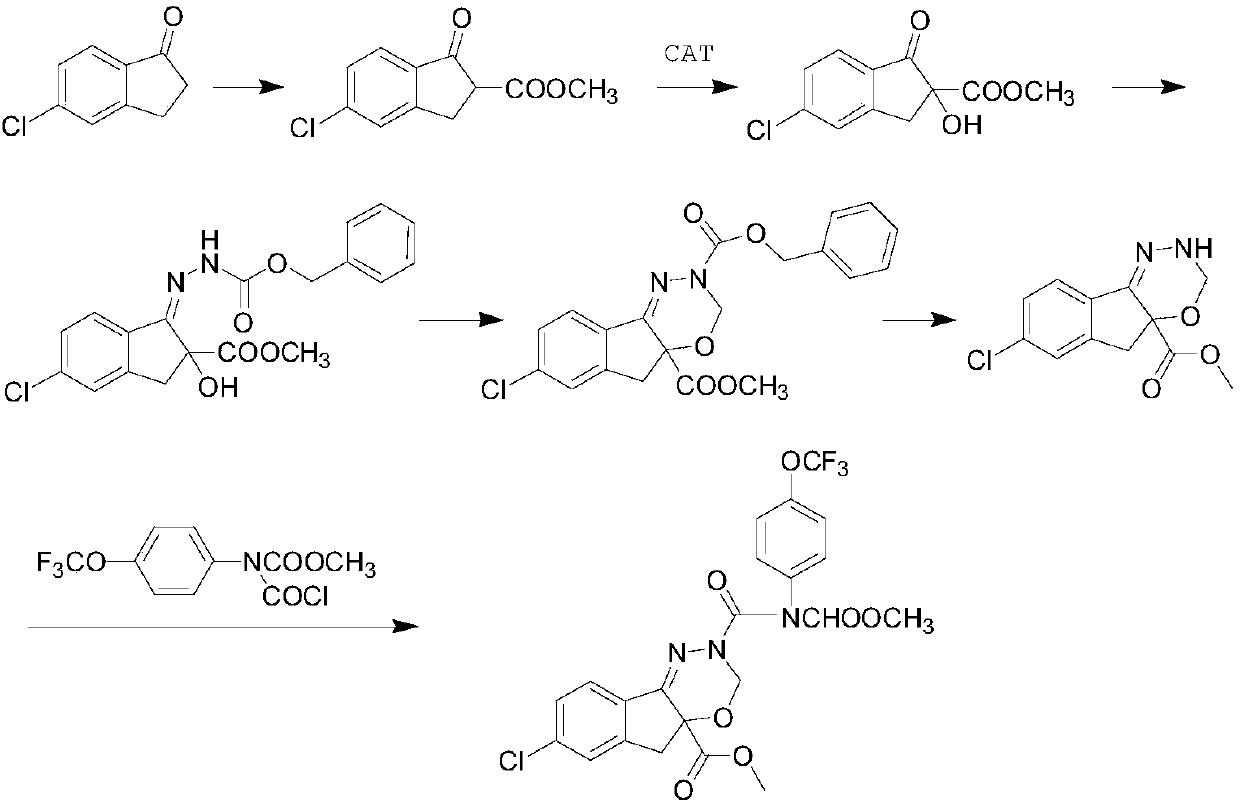
[0053] Embodiment 3. Recycling of metal polymer catalyst
[0054] The catalyst recovered by filtration in Example 2 was added to toluene, and then 113g of tert-butyl hydroperoxide and 224g of 5-chloro-2-methoxycarbonyl-1-indanone ester and 1200mL of toluene were added to carry out the asymmetric synthesis reaction, The temperature was raised to 110° C. for reflux reaction for 4 hours. After the reaction is completed, the metal polymer catalyst is recovered by filtration, and the obtained filtrate can be distilled under reduced pressure to obtain the key intermediate 5-chloro-2-methoxycarbonyl-2-hydroxyl-1-indanone; at the same time, the recovered catalyst is put into 50mL A suspension is formed in toluene solvent to continue the asymmetric synthesis reaction; the prepared intermediate is prepared according to the conventional prior art to obtain the S body indoxacarb.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com