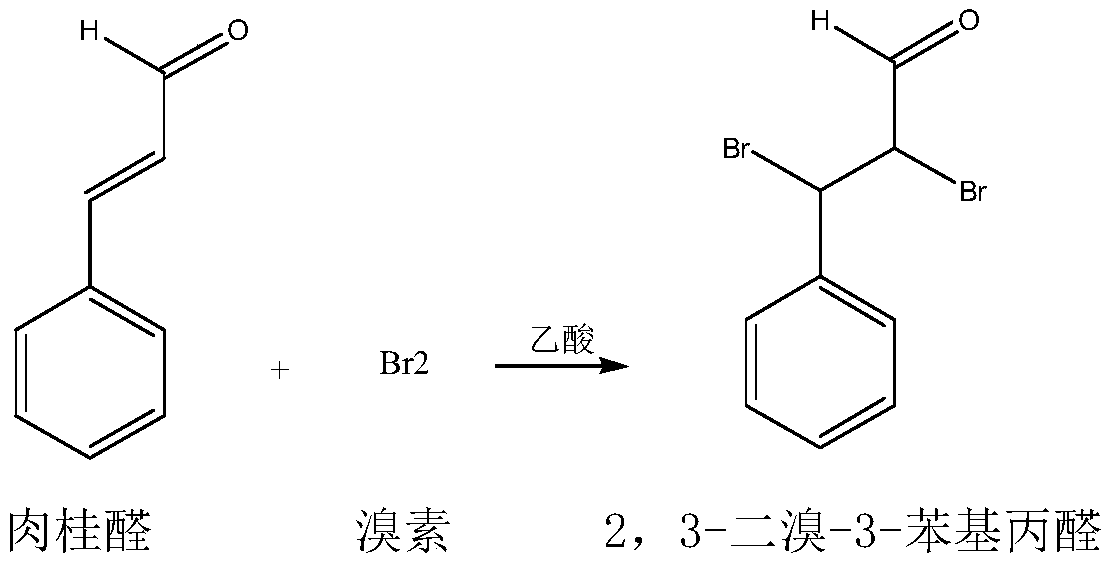
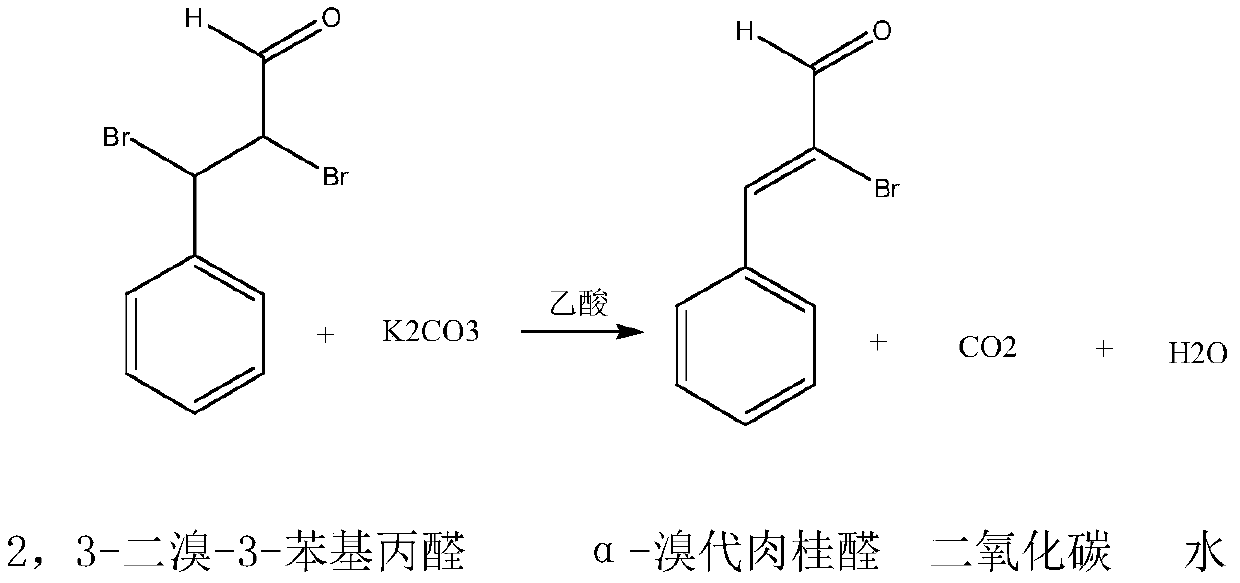
Synthetic method for 2-bromo-3-phenyl-2-propenal
A technology of bromocinnamaldehyde and synthesis method, which is applied in the field of synthesis of fine chemical product α-bromocinnamaldehyde, can solve the problems of high raw material cost, complicated post-treatment process, low yield, etc., and achieve low raw material cost and three wastes The effect of small quantity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of silica gel supported bromine:
[0038] Drop into bromine in the clean 1000ml three-necked flask that has separator, reflux condenser (reflux condenser is installed on the top of separator), stirring paddle, thermometer: 18.0g (content: 99.2%, 0.1116mol), ring Hexane: 600ml. The outer wall of the three-necked flask is slowly heated with an electric heating mantle, and the cyclohexane vaporizes and enters the condenser for cooling, and the condensate flows into the water separator. At the beginning, the cyclohexane deposited in the water separator became turbid and small water droplets were deposited at the bottom of the water separator. After refluxing for a period of time, the cyclohexane returned to the water separator became clear, and the water separation was completed.
[0039] After the water separation is completed, stop heating and let it cool down naturally. Add 35 g of silica gel to the three-necked flask, and stir slowly for 30 minutes. Chang...
Embodiment 2
[0050] Preparation of silica gel supported bromine:
[0051] Drop into bromine in the clean 1000ml three-necked flask that has separator, reflux condenser (reflux condenser is installed on the top of separator), stirring paddle, thermometer: 15.3g (content: 99.2%, 0.0949mol), ring Hexane: 600ml. The outer wall of the three-necked flask is slowly heated with an electric heating mantle, and the cyclohexane vaporizes and enters the condenser for cooling, and the condensate flows into the water separator. At the beginning, the cyclohexane deposited in the water separator became turbid and small water droplets were deposited at the bottom of the water separator. After refluxing for a period of time, the cyclohexane returned to the water separator became clear, and the water separation was completed.
[0052] After the water separation is completed, stop heating and let it cool down naturally. Add the silica gel filtered out from the previous batch into the three-necked flask, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com