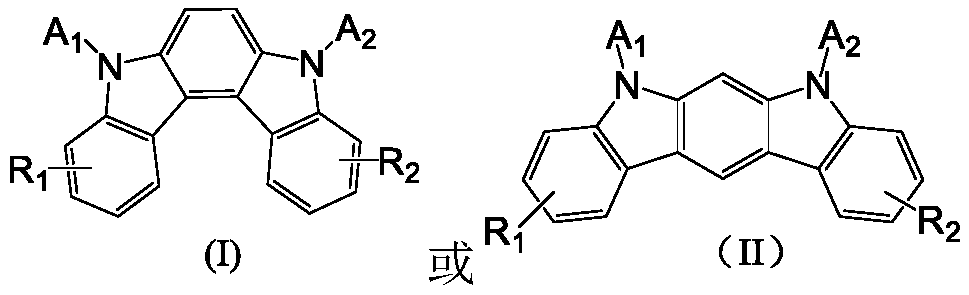
Bipolar thermal delayed fluorescence compound, and applications thereof
A fluorescent compound and thermal delay technology, applied in the fields of photovoltaic power generation, luminescent materials, organic chemistry, etc., can solve the problems of improving or reducing the intensity of the photoradiation transition constant array, unfavorable molecular luminous efficiency, etc., to increase the crossover probability and improve luminescence The effect of efficiency and device stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] The specific embodiment of the present invention also provides the above-mentioned preparation method, which is synthesized through the following general synthetic route:
[0071]
[0072] in,
[0073] Pd(PPh 3 ) 4 Is tetrakis (triphenylphosphine) palladium;
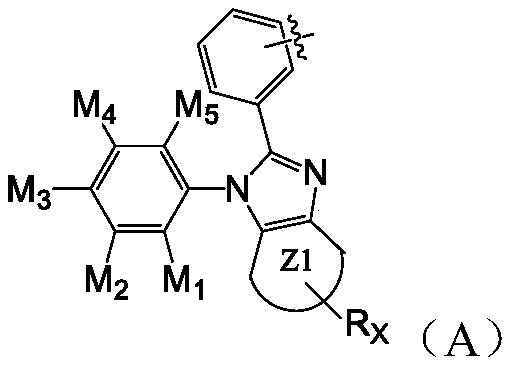
[0074] R 1 , R 2 Each is independently a hydrogen atom, a deuterium atom, a C1-C8 straight chain or branched chain alkyl group, an unsubstituted or C6-C36 aryl group substituted by a C1-C8 straight chain alkyl group, an unsubstituted or C1-C8 straight chain alkyl group, Alkanyl substituted C5-C30 heteroaryl;
[0075] Z1 ring is selected from unsubstituted or C6-C36 aryl substituted by C1-C8 linear alkyl;
[0076] R X selected from hydrogen atom, deuterium atom, C1-C8 straight chain or branched chain alkyl, C6-C36 aryl unsubstituted or substituted by C1-C8 straight chain alkyl, unsubstituted or C1-C8 straight chain alkane C5-C30 heteroaryl substituted by base; R X 0-4;
[0077] m 1 -M 5 Each is indep...
Embodiment 1
[0080] The preparation of embodiment 1:L2
[0081]
[0082] After adding a certain amount of D2 and K2 into the three-necked flask, install a mechanical stirring bar, feed nitrogen for 20 minutes, and add catalyst Pd (PPh 3 ) 4 0.25-3mol%, 0.018mol of 2M alkali solution, heated to reflux, reacted for 5-10 hours, suction filtered after reaction, washed with toluene, washed with ethanol, and recrystallized in xylene to obtain L2 powder with a purity of more than 99%. In order to further improve the purity of L2, a vacuum sublimation apparatus is used to carry out one or more sublimation, and an L2 product with a purity greater than 99.5% can be obtained.
[0083] Using CDCL 3 Used as a solvent, tetramethylsilane (δ = 0.00ppm) as an internal standard record 1 H NMR spectrum.
[0084] 1 H NMR (400MHZ, DMSO-d6):
[0085] 7.00-7.08ppm (4H, m), 7.24-7.30ppm (10H, m), 7.40-7.55ppm (16H, m), 7.70ppm (4H, m).
Embodiment 2
[0086] The preparation of embodiment 2:L4
[0087]
[0088] After adding a certain amount of D4 and K4 into the three-necked flask, install a mechanical stirring rod, feed nitrogen for 20 minutes, and add catalyst Pd (PPh 3 ) 4 0.25-3mol%, 0.018mol of 2M alkali solution, heated to reflux, reacted for 5-10 hours, suction filtered after reaction, washed with toluene, washed with ethanol, and recrystallized in xylene to obtain L4 powder with a purity of more than 99%. In order to further improve the purity of L4, a vacuum sublimation apparatus is used to carry out one or more sublimation, and an L4 product with a purity greater than 99.5% can be obtained.
[0089] Using CDCL 3 Used as a solvent, tetramethylsilane (δ = 0.00ppm) as an internal standard record 1 H NMR spectrum.
[0090] 1 H NMR (400MHZ, DMSO-d6):
[0091] 7.00-7.08ppm(4H,m),7.24-7.30ppm(10H,m),7.40ppm(2H,d),7.50ppm(4H,d),7.55ppm(2H,d),7.70-7.80ppm(10H ,m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com