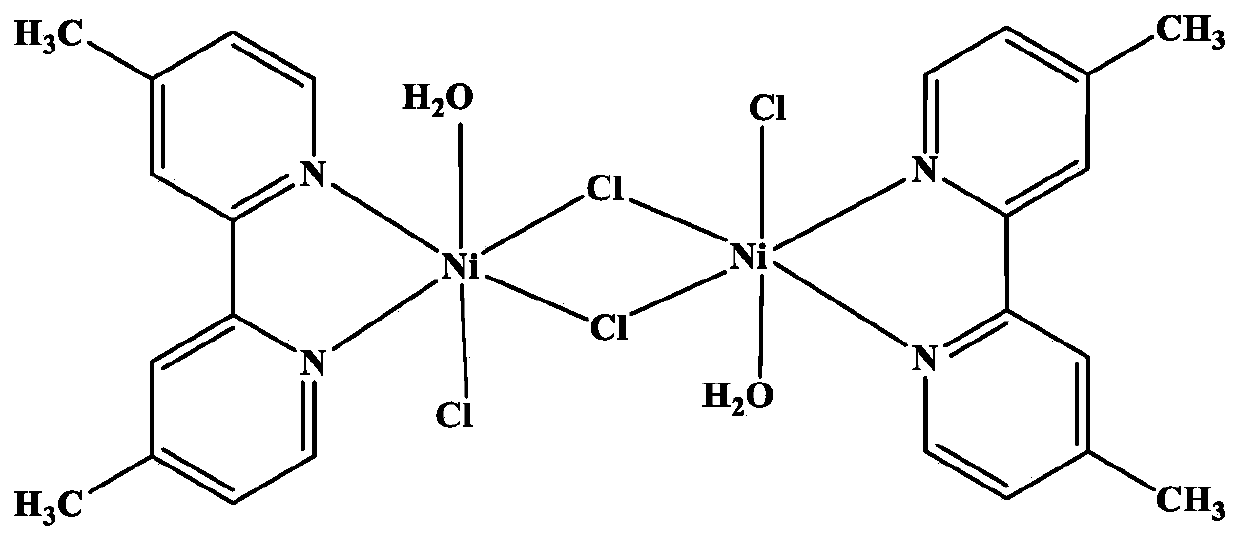
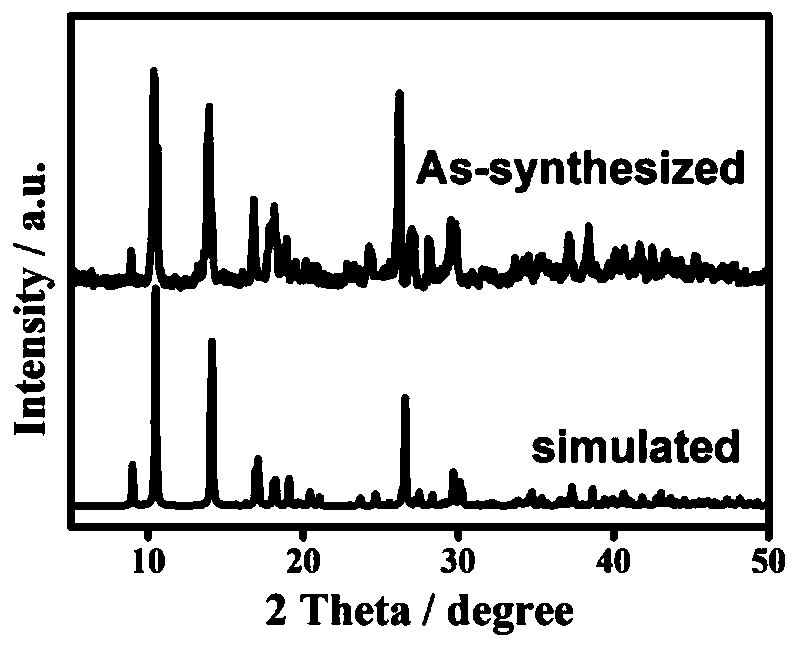
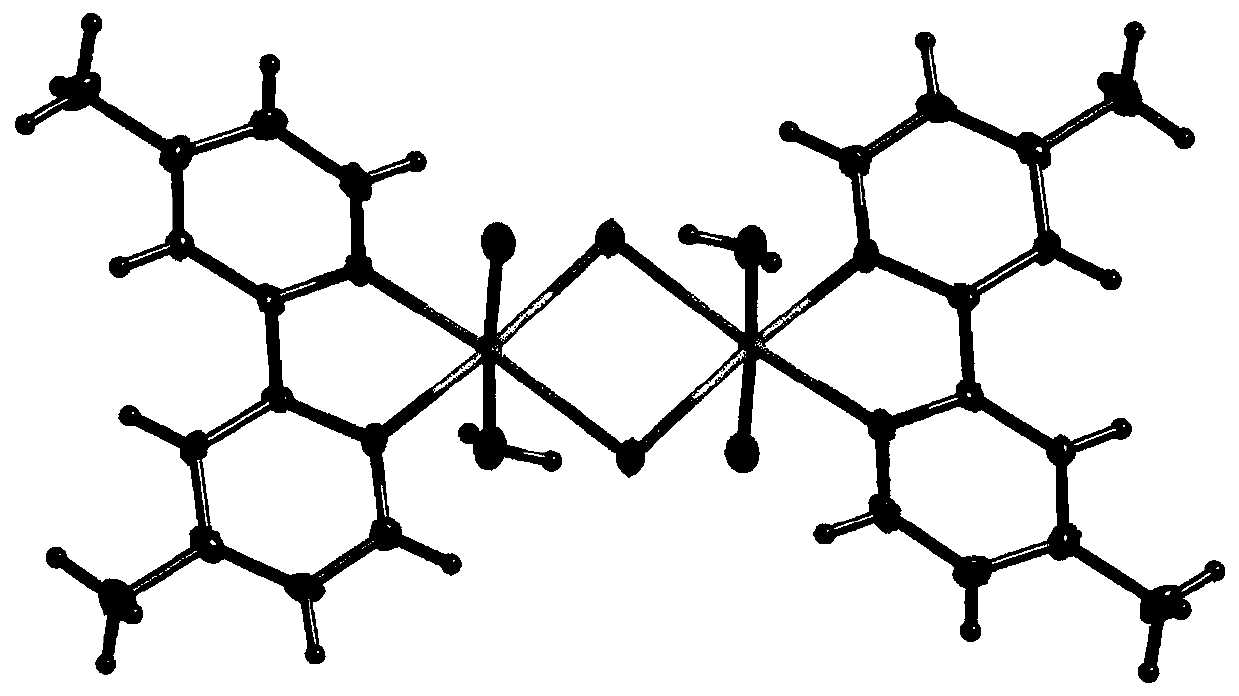
Dinuclear nickel coordination compound and preparation method and application thereof
A coordination compound and coordination technology are applied in the fields of binuclear nickel coordination compounds and their preparation, and binuclear metal coordination compounds and their preparation fields, which can solve the problems of difficult large-scale industrial production, harsh synthesis conditions, high prices and the like, Achieve the effect of high yield, simple preparation and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 36.65 mg (0.2 mmol) of 4,4'-dimethyl-2,2'-bipyridine and 131.24 mg (0.2 mmol) of triphenylphosphine nickel dichloride into 50 mL of acetonitrile and mix well; Argon gas was introduced into the solution, and after stirring and reacting at 50° C. for 20 hours, a green precipitate was obtained; the above precipitate was dissolved with a mixed solution of acetonitrile and ethanol, wherein the volume of acetonitrile was 50%. After slowly volatilizing for 2 weeks, a green single crystal sample was obtained.
Embodiment 2
[0034] Add 36.65 mg (0.2 mmol) of 4,4'-dimethyl-2,2'-bipyridine and 65.62 mg (0.1 mmol) of triphenylphosphine nickel dichloride into 40 mL of acetonitrile and mix well; Argon was introduced into the solution, and after stirring and reacting at 60° C. for 15 hours, a green precipitate was obtained; the above precipitate was dissolved with a mixed solution of acetonitrile and ethanol, wherein the volume of acetonitrile was 50%. After slowly volatilizing for 2 weeks, a green single crystal sample was obtained.
Embodiment 3
[0036] Add 36.65 mg (0.2 mmol) of 4,4'-dimethyl-2,2'-bipyridine and 98.28 mg (0.15 mmol) of triphenylphosphine nickel dichloride into 60 mL of acetonitrile and mix well; Argon gas was introduced into the liquid, and after stirring and reacting at 70° C. for 15 hours, a green precipitate was obtained; the above precipitate was dissolved with a mixed solution of acetonitrile and ethanol, wherein the volume of acetonitrile was 60%. After slowly volatilizing for 2 weeks, a green single crystal sample was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com