Antitumor lymphatic metastasis function of mitoxantrone and pharmaceutical preparation of mitoxantrone
A technology for mitoxantrone and pharmaceutical preparations, applied in the field of medicine, can solve problems such as toxic and side effects, and achieve the effects of reducing toxic and side effects, reducing the risk of tumor recurrence, and inhibiting tumor lymphatic metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the preparation of solution
[0025] This embodiment provides a mitoxantrone solution, and the proportion of the raw materials per 100ml of the drug is as follows: 0.5% mitoxantrone, 0.01% sodium acetate, 0.02% sodium metabisulfite, 0.092% acetic acid, and 0.8% sodium chloride. The preparation process is as follows: 0.01% sodium acetate, 0.02% sodium pyrosulfite, 0.092% acetic acid and 0.8% sodium chloride are dissolved in 100ml of pure water to obtain a solution for injection. Add 0.5% mitoxantrone to the solution for injection, stir magnetically at 50 rpm at room temperature for 25 minutes, then add medicinal charcoal, stir magnetically at 50°C and 50 rpm for 20 minutes to obtain a mitoxantrone solution. The mitoxantrone solution was filtered through a 0.22 μm microporous membrane, and sterilized at 121° C. for 15 minutes to obtain a sterilized mitoxantrone solution.
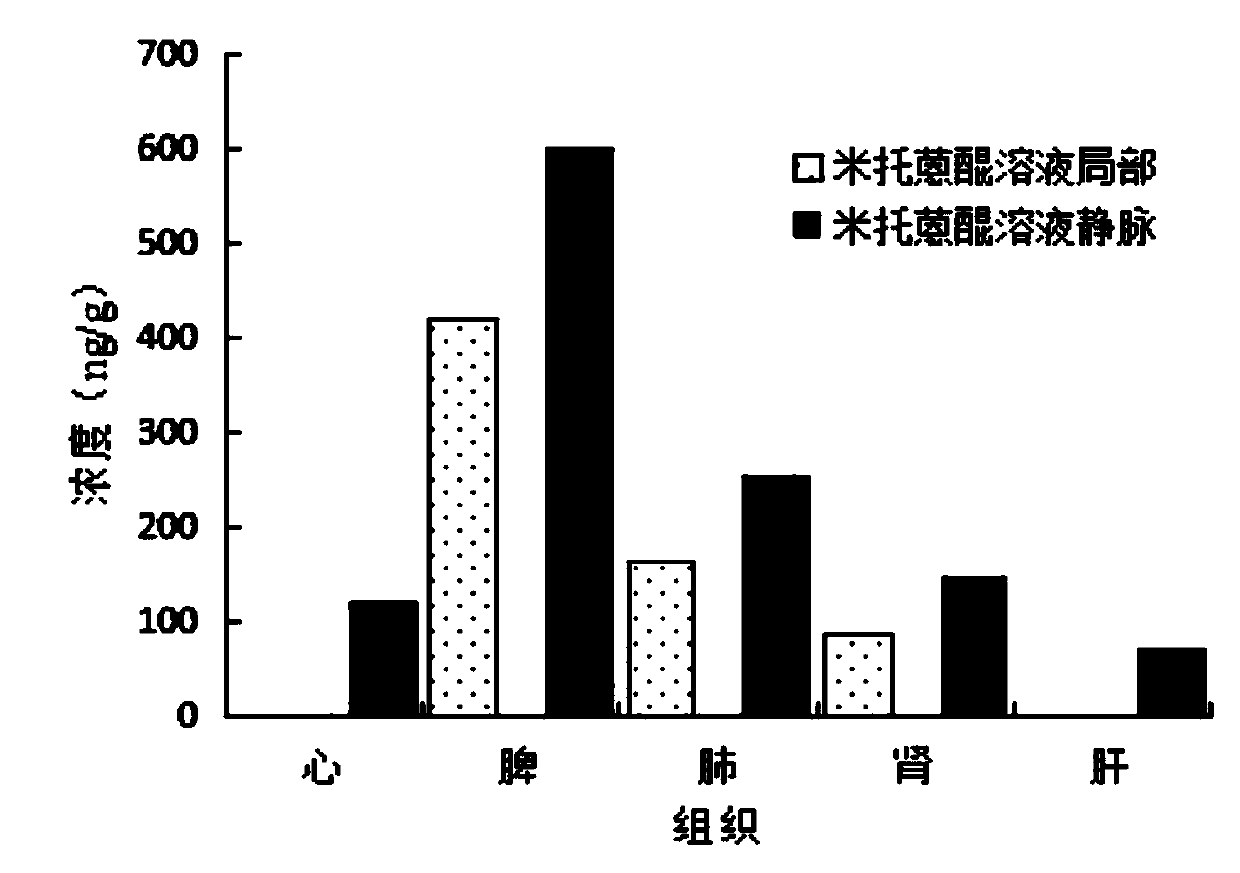
[0026] In this embodiment 1, in order to further verify the beneficial effects of the...
Embodiment 2
[0044] Embodiment 2, the preparation of microcrystalline gel
[0045] The present embodiment provides a mitoxantrone microcrystalline gel, and the ratio of each raw material per 10 g of the medicine is as follows: 10 mg of mitoxantrone, 5 mg of mitoxantrone hydrochloride, 2 g of poloxamer 407, and 2 g of poloxantrone Mu 188 0.5g, 7.49ml distilled water. The preparation process is as follows: accurately weigh 10 mg of mitoxantrone and 5 mg of mitoxantrone hydrochloride after grinding and sieving (200 mesh), add 2 g of Poloxamer 407, 0.5 g of Poloxamer 188, and 7.49 ml of distilled water , stirred magnetically at 30 rpm in an ice-water bath for 6 hours until the particles were completely dissolved, and placed at 4°C for 24 hours to obtain mitoxantrone microcrystalline gel.
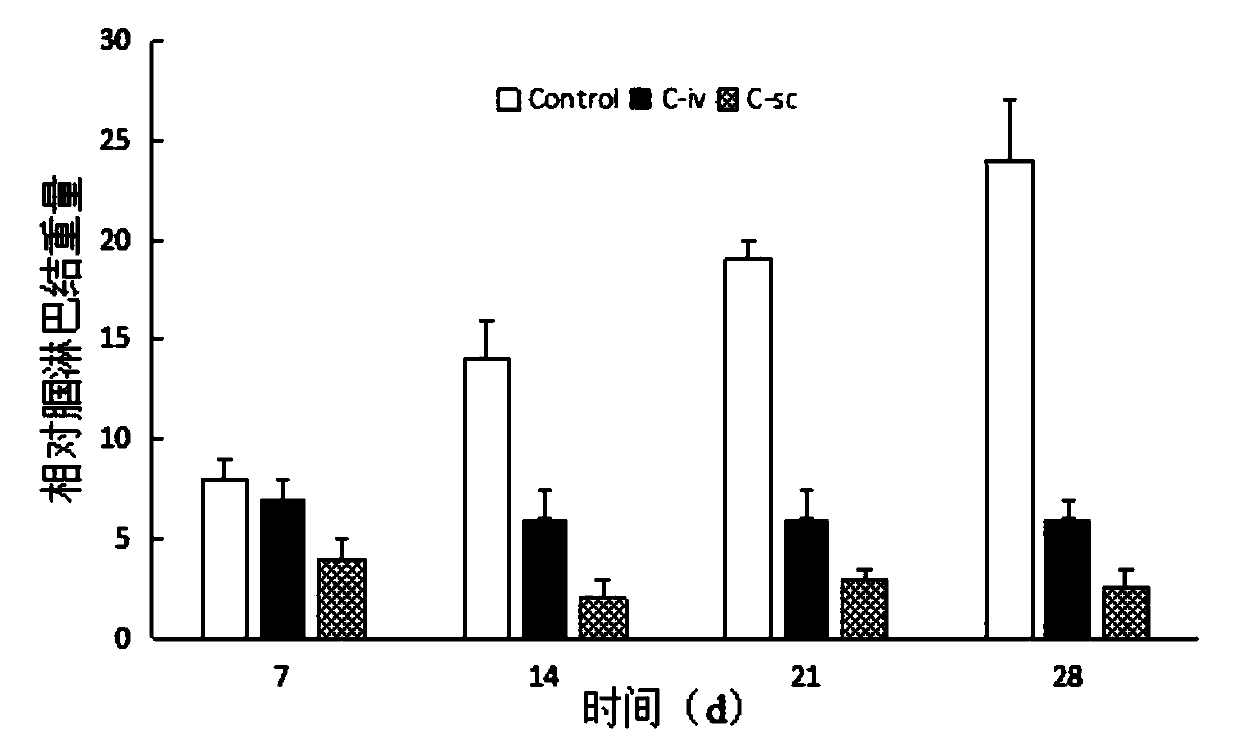
[0046] In order to verify that the microcrystalline gel in Example 2 can reduce the drug toxicity of systemic administration of mitoxantrone after local administration, this example investigated the tissue ...
Embodiment 3
[0047] Embodiment 3, the preparation of liposome
[0048] This embodiment provides a mitoxantrone liposome, and the ingredients of each 10ml drug are formulated as follows: mitoxantrone 10mg, soybean lecithin 0.4g, cholesterol 0.04g, and glucose 1.5g. The preparation process is as follows: add 10 mg of mitoxantrone, 0.4 g of soybean lecithin, and 0.04 g of cholesterol solution to an appropriate amount of absolute ethanol to obtain a clear solution. Add 1.5 g of glucose powder to the clear solution, shake well, and sonicate to a milky white suspension. Ethanol was removed from the milky white suspension by rotary evaporation at 50°C to obtain a liposome film. Add 10ml of distilled water to the liposome film and hydrate at 50°C until all the solids are dissolved. The probe is sonicated for 10 minutes, and the mitoxantrone liposome is obtained by passing through a 0.45 μm filter membrane.
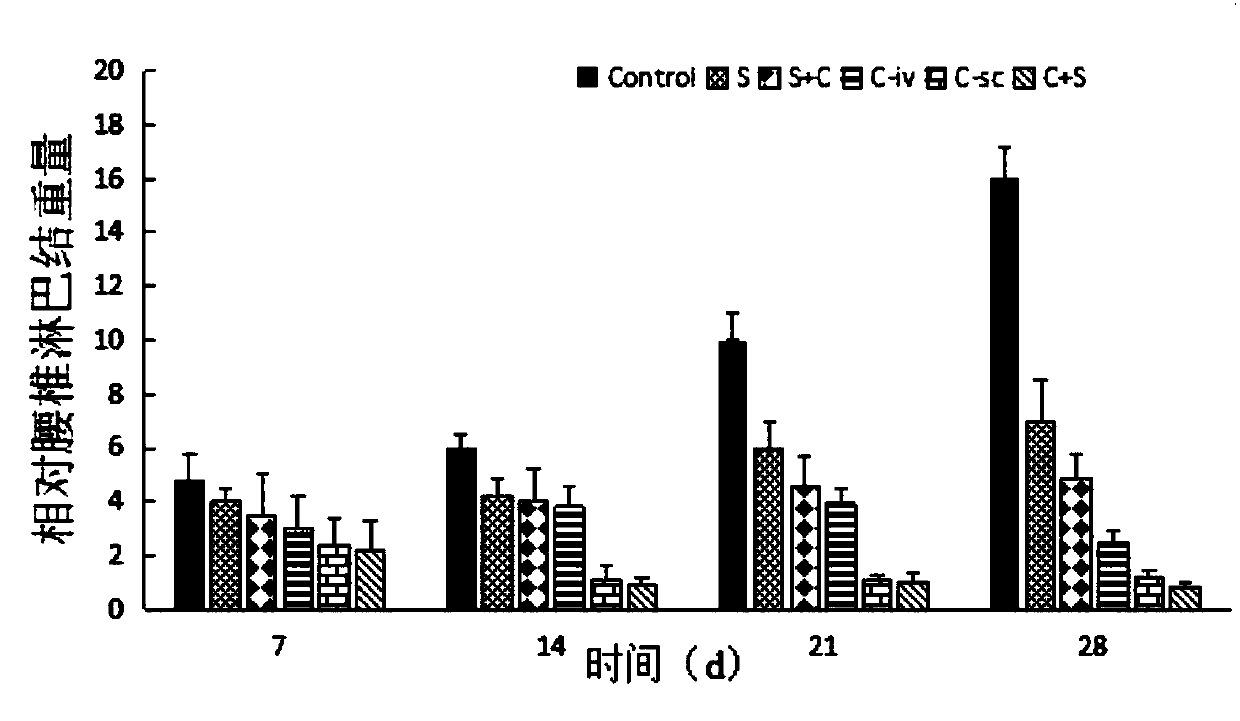
[0049] In order to verify that the liposomes in Example 3 can reduce the drug toxicity o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com