A method and device system for recovering nitric acid by nitrate pyrolysis
A nitrate and preheating device technology, which is applied in the direction of nitric acid, nitrogen oxides/oxo acids, sustainable manufacturing/processing, etc., can solve the problems of high energy consumption and poor efficiency of the device, and achieve avoiding direct contact and recycling High utilization rate and good decomposition reaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Nitrate pyrolysis to recover nitric acid
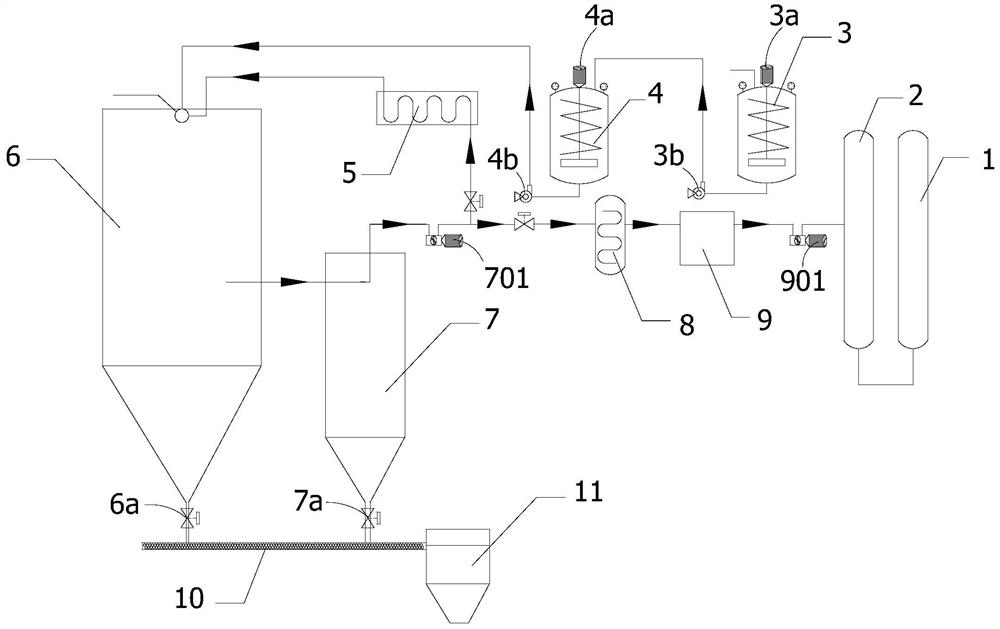
[0046] The crystalline nitrate is heated using a two-stage preheating device. The crystalline nitrate is first heated to about 170°C in the first stage preheating device and converted into liquefied nitrate. Then it is transported to the second-stage preheating device and heated to about 330°C, which is 500°C lower than the decomposition temperature, to obtain a nitrate thermal fluid with a temperature of about 330°C. The nitrate hot fluid is transported to the decomposer and heated with high-temperature gas (temperature 550-800℃), and the internal temperature of the decomposer is maintained at 500-800℃, so that the nitrate decomposes to produce mixed gas and solid powder. Separate the mixed gas from the solid powder, part of the mixed gas is transported to the nitric acid recovery tank to be absorbed by water and converted into nitric acid, and the other part of the mixed gas is heated to 500-800°C, and then returned to the deco...
Embodiment 2
[0048] Nitrate pyrolysis to recover nitric acid
[0049] According to the thermal decomposition characteristics of nitrate: firstly, heat the nitrate once, raise the temperature to 150~200℃ and keep the temperature to convert the nitrate into a liquid state. Then, the liquid nitrate is pumped into the secondary heating tank through the transfer pump, and the nitrate temperature reaches 250~350℃ to obtain the nitrate thermal fluid.
[0050] The nitrate thermal fluid (liquid) is pumped into the nitrate decomposer. At the inlet of the pump into the decomposer, there is a liquid mist distributor, so that the pumped nitrate liquid is distributed in mist. The internal temperature of the decomposer is controlled to be maintained between 500 and 800 ℃, and the pumped nitrate liquid is decomposed into nitrogen dioxide, oxygen and oxides. Oxide powder is deposited on the bottom of the decomposer. There is a sealed valve at the bottom of the decomposer. After the oxide powder is accumulated...
Embodiment 3
[0063] The device system for nitrate pyrolysis and recovery of nitric acid includes heating tank, decomposer, dust collector, heater, heat exchanger, and nitric acid recovery tank. The heating tank is used for heating and melting the nitrate to obtain the nitrate hot fluid. The decomposer is used to receive the nitrate thermal fluid melted by the heating tank and the mixed gas heated by the heater; the nitrate is thermally decomposed inside the decomposer to generate oxides and mixed gas. The dust collector is used to receive the decomposed mixed gas discharged from the decomposer, and separate dust entrained in the mixed gas. The heater is used to receive the dust-separated mixed gas discharged from the dust collector, heat the mixed gas and deliver it to the feed inlet of the decomposer as a heat source. The heat exchanger is used to receive the dust-separated mixed gas discharged from the dust collector, cool and transport it to the nitric acid recovery tank.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com