Preparation method for fluoroquinolones intermediate 2,4-dichloro-5-fluorobenzoyl chloride
A technology of fluorobenzoyl chloride and fluoroquinolones, applied in the field of drug synthesis, can solve the problems of unfavorable industrialized production, hidden safety hazards and high cost, and achieve the effects of reducing production risks, reducing pollution and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
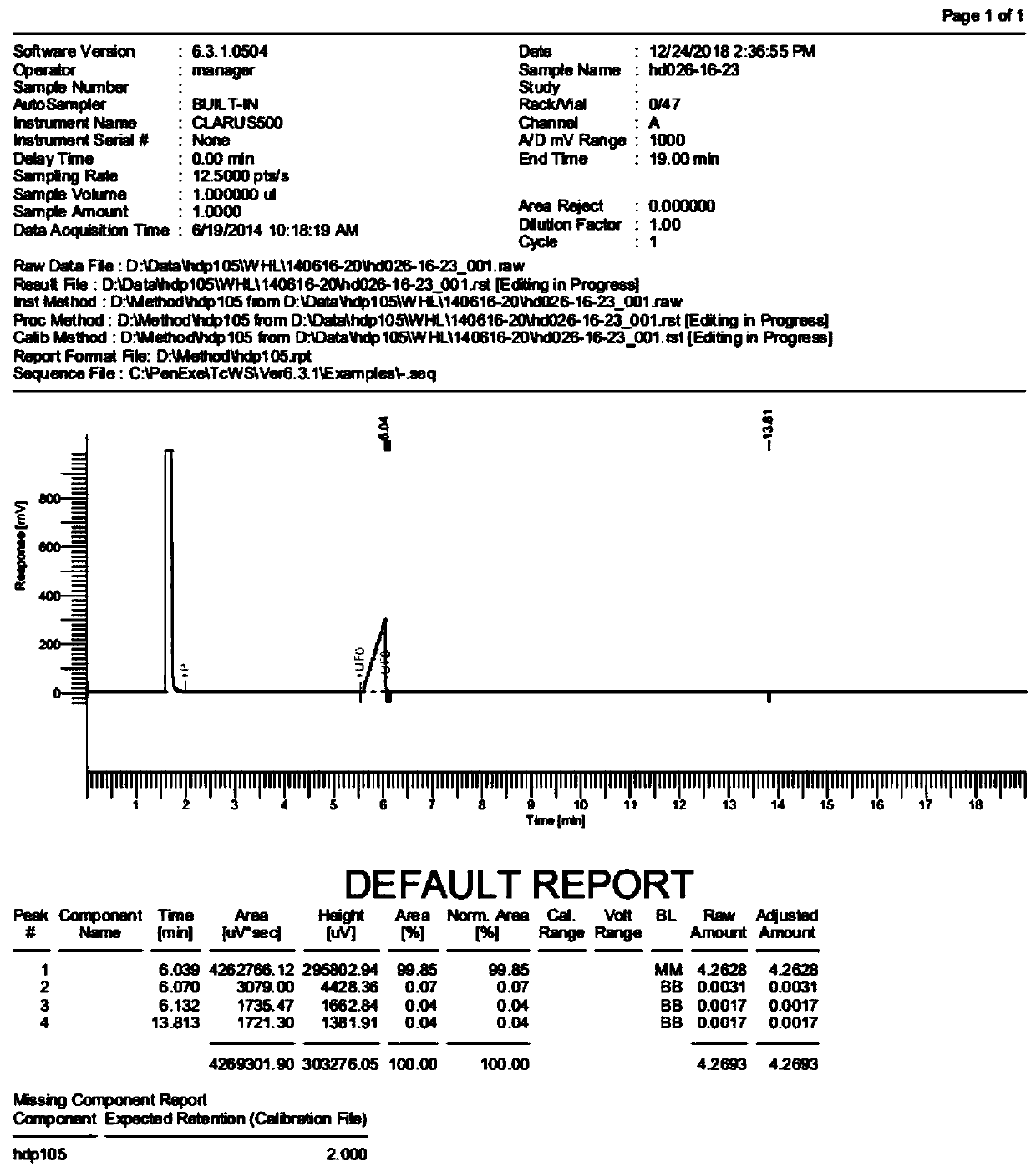
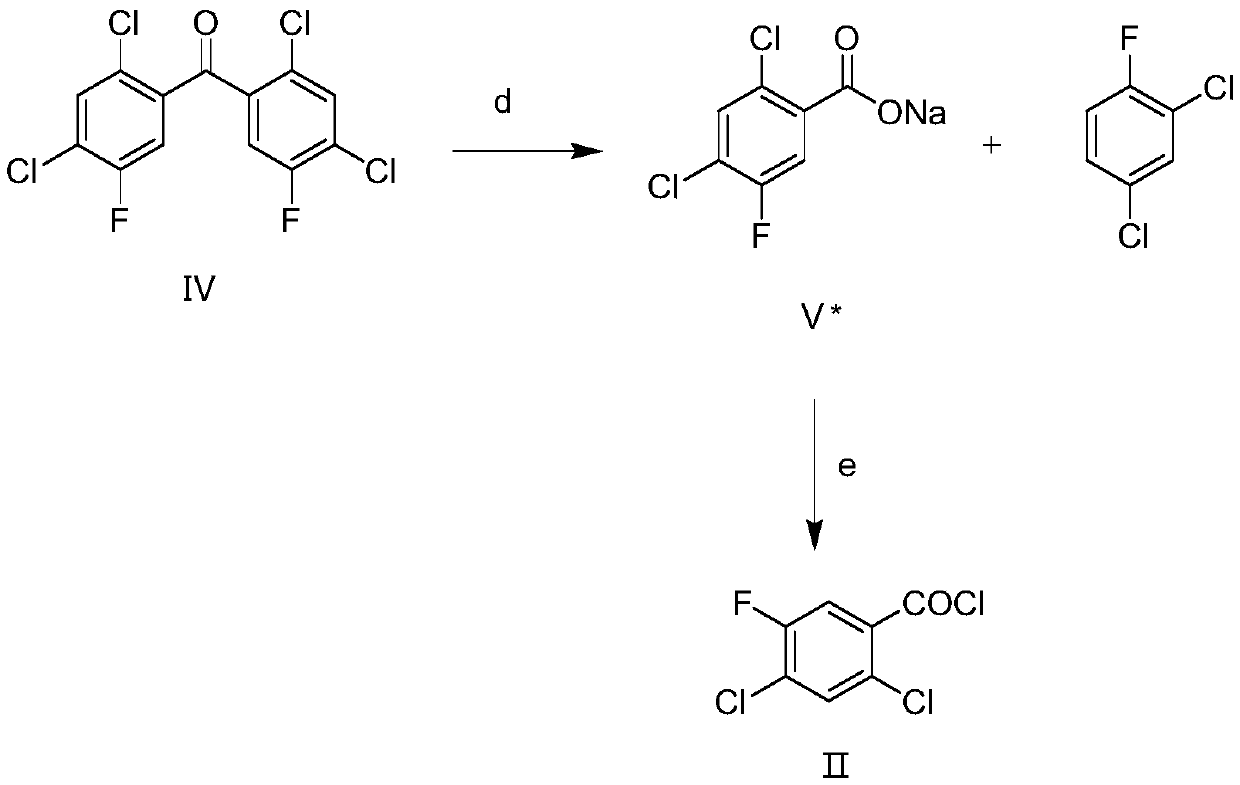
[0043] The preparation method of this fluoroquinolone intermediate 2,4-dichloro-5-fluorobenzoyl chloride comprises the steps:
[0044] Step 1: Alkaline hydrolysis reaction
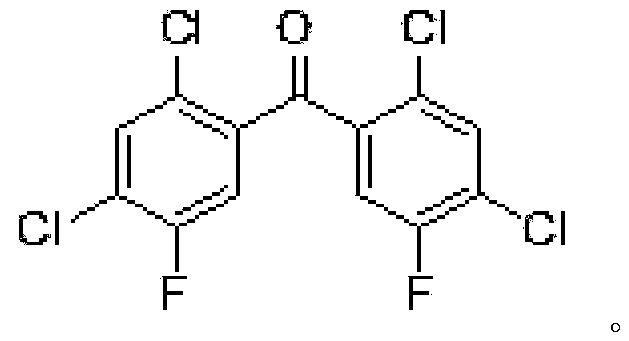
[0045] Heating the prepared compound (IV) into a molten state, adding an alkaline reagent, maintaining the molten state, stirring for reaction, adding a solvent, refluxing, and fully reacting to obtain compound V* and 2,4-dichlorofluorobenzene;
[0046] Step 2: Acylation reaction
[0047] Control the temperature at 49-51°C, slowly add sulfone dichloride dropwise to compound V*, raise the temperature to 69-71°C, stir and react to obtain compound 2,4-dichloro-5-fluorobenzoyl chloride.
[0048] In this example, this example provides a method for converting compound 2,4-dichlorofluorobenzene into compound II, comprising the following steps:
[0049] Step 1: Add raw material 2,4-dichlorofluorobenzene (100g, 0.606mol) into carbon tetrachloride (107.4g, 0.667mol), stir and dissolve, then add ferric chloride (1....
Embodiment 1
[0055] Add compound IV (100g) to the reaction flask, keep the temperature at 90-100°C, compound IV is in molten state, add sodium hydroxide (1.5eq, 16g), keep the temperature at 90-100°C, stir for 1 hour , Let the sodium hydroxide fully disperse, slowly raise the temperature to about 110°C, the system will react and release heat by itself, and the temperature will rise to 140-150°C, the temperature of the water bath is controlled at 120-130°C, at this time, HPLC detects that the remaining raw materials are 10-30% , lower the temperature to about 110°C, add 1,4-dioxane (100ml) at one time, reflux for about 3 hours, HPLC detects that the remaining raw materials are less than 1%, distill at atmospheric pressure, remove dioxane and water, and obtain a mixture compound V* and 2,4-dichlorofluorobenzene;
[0056] In the above mixture (compound V* and 2,4-dichlorofluorobenzene), keep the temperature at about 50°C, slowly add thionyl chloride (1.3eq, 42g) dropwise, raise the temperatur...
Embodiment 2
[0058] Add compound IV (500g) to the reaction flask, keep the temperature at 90-100°C, compound IV is in molten state, add sodium hydroxide (1.5eq, 80.4g), keep the temperature at 90-100°C, stir for 1 hour , Let the sodium hydroxide fully disperse, slowly raise the temperature to about 110°C, the system will react and release heat by itself, and the temperature will rise to 140-150°C, the temperature of the water bath is controlled at 120-130°C, at this time, HPLC detects that the remaining raw materials are 10-30% , lower the temperature to about 110°C, add 1,4-dioxane (500ml) at one time, reflux for about 3 hours, HPLC detects that the remaining raw materials are less than 1%, distill at atmospheric pressure, remove dioxane and water, and obtain a mixture compound V* and 2,4-dichlorofluorobenzene;
[0059] In the above mixture (compound V* and 2,4-dichlorofluorobenzene), keep the temperature at about 50°C, slowly add thionyl chloride (1.3eq, 207g) dropwise, raise the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com