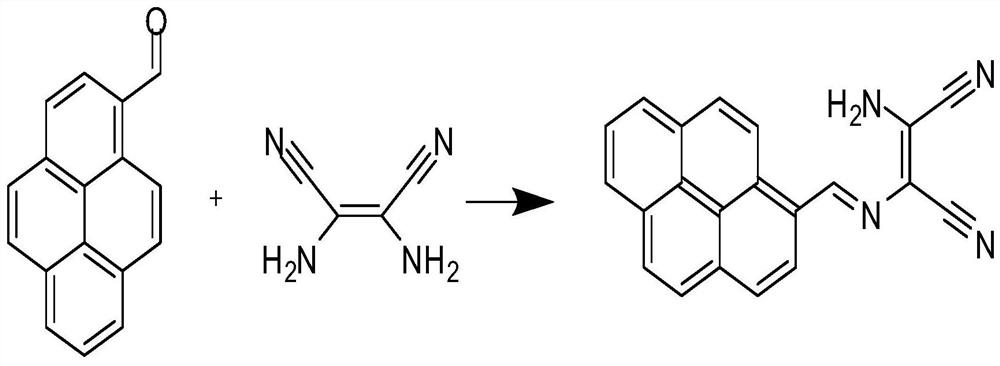
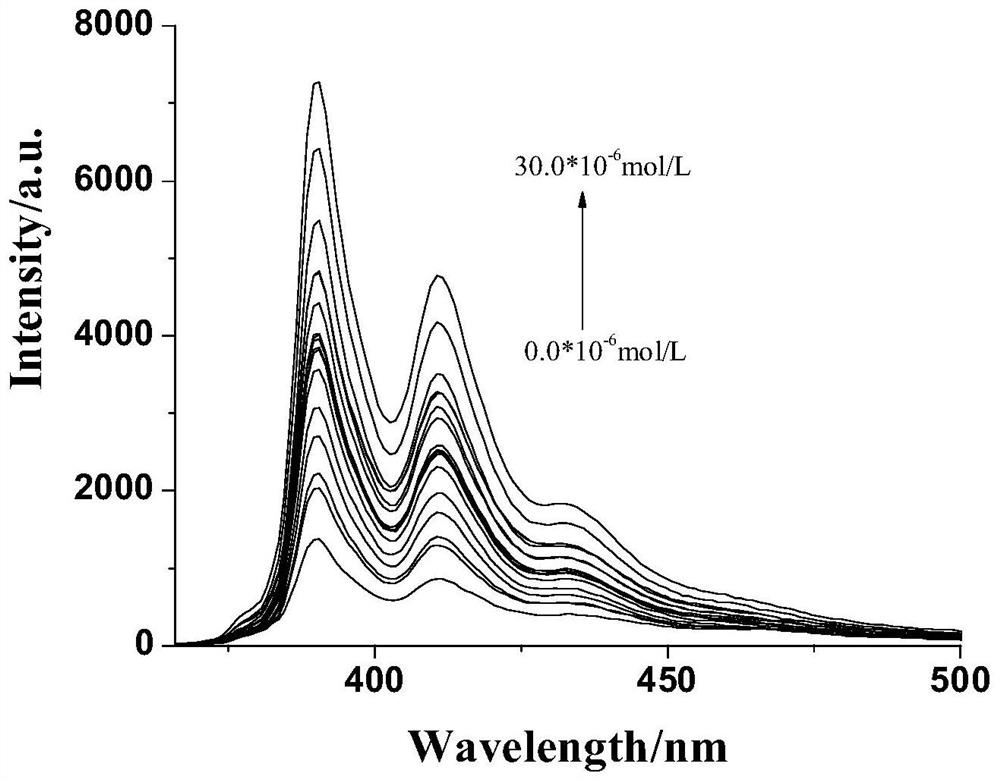
A kind of compound 2-amino-3-(pyrene-1-methyleneamino)maleonitrile and its preparation method and application
A technology of methyleneamino and maleonitrile, which is applied to the compound 2-amino-3-(pyrene-1-methyleneamino)maleonitrile and its preparation and application fields, can solve equipment or sample preparation troubles, unsuitable S real-time detection, affecting sulfur ion detection and other issues, to achieve stable fluorescence, good accuracy, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 2-amino-3-(pyrene-1-methyleneamino)maleonitrile:
[0033] 1) Add 1mmol pyrene formaldehyde to the round bottom flask, then add the same amount of diaminomaleonitrile, then add enough methanol to the flask to dissolve the reactants, and heat at 60°C for 6h, confirm the progress of the reaction by spotting the plate completely;
[0034] 2) After the reaction, the solution was slowly poured into ice water, then filtered, washed 3 times with ice ethanol, dried, and the obtained crude product was separated and purified by column chromatography with 1:1 petroleum ether and dichloromethane, 2-amino-3-(pyrene-1-methyleneamino)maleonitrile can be obtained.
Embodiment 2
[0036] Preparation of 2-amino-3-(pyrene-1-methyleneamino)maleonitrile:
[0037] 1) Add 1mmol pyrene formaldehyde to the round bottom flask, then add the same amount of diaminomaleonitrile, then add enough methanol to the flask to dissolve the reactants, and heat at 60°C for 4h, confirm the progress of the reaction by spotting the plate completely;
[0038] 2) After the reaction, the solution was slowly poured into ice water, then filtered, washed 3 times with ice ethanol, dried, and the obtained crude product was separated and purified by column chromatography with 1:1.8 petroleum ether and dichloromethane, 2-amino-3-(pyrene-1-methyleneamino)maleonitrile can be obtained.
Embodiment 3
[0040] Preparation of 2-amino-3-(pyrene-1-methyleneamino)maleonitrile:
[0041] 1) Add 1mmol pyrene formaldehyde to the round bottom flask, then add the same amount of diaminomaleonitrile, then add enough methanol to the flask to dissolve the reactant, and heat at 60°C for 5h, confirm the progress of the reaction by spotting the plate completely;
[0042] 2) After the reaction, the solution was slowly poured into ice water, then filtered, washed 3 times with ice ethanol, dried, and the obtained crude product was separated and purified by column chromatography with 1:0.5 petroleum ether and dichloromethane, 2-amino-3-(pyrene-1-methyleneamino)maleonitrile can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com