Preparation method of nitrendipine
A technology of nitrendipine and ethyl nitrobenzylidene acetoacetate is applied in the field of medical technology, can solve the problems of not paying attention to transesterification impurities and the like, and achieve the effects of easy industrial application, lowering reaction temperature and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
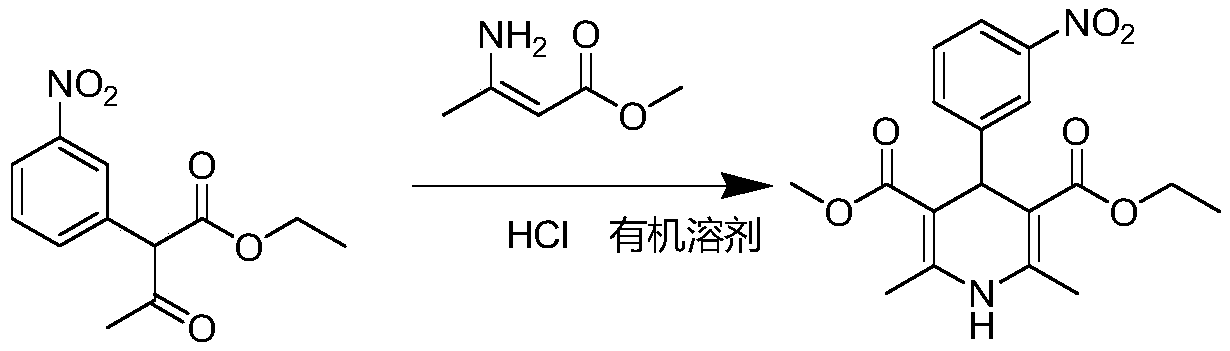
[0043] Step (1): Pump 375.8g of weighed absolute ethanol into a 1000ml reactor, then add 140g of ethyl 3-nitrobenzylidene acetoacetate and 61.2g of methyl 3-aminobutenoate, and turn on Stir.
[0044] Step (2): After the feeding is completed, the temperature is raised to 70~75℃, and the reaction is kept at this temperature for 1h; then, the temperature is controlled at 70~75℃, and 6.4g of concentrated hydrochloric acid is slowly added. After the addition is completed, it is carried out by TLC immediately Control (developing agent: PE:EA=3:1);
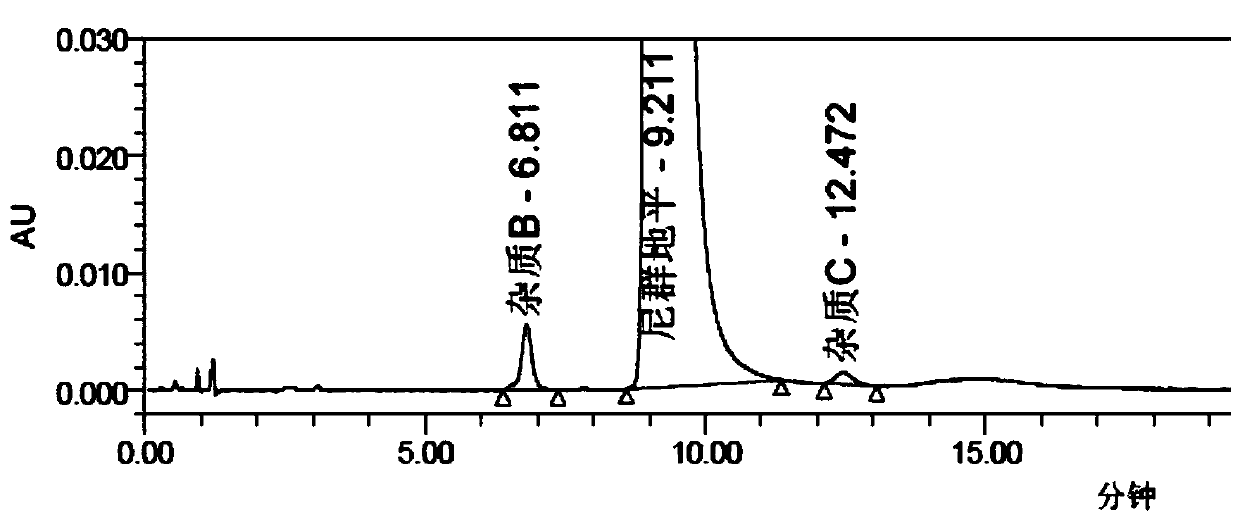
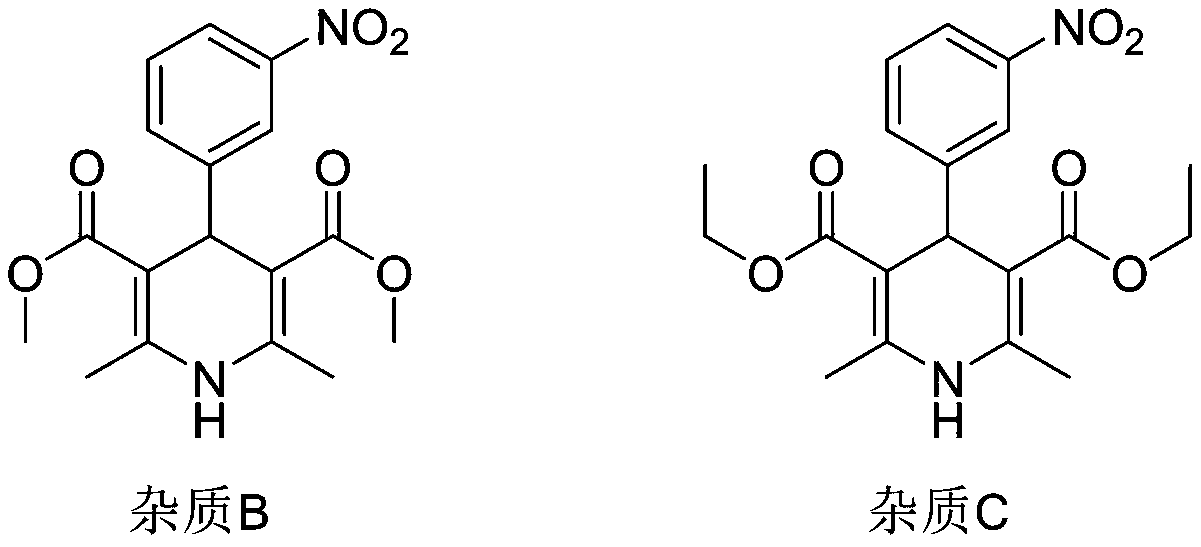
[0045] Step (3): Immediately (within 10 minutes) after TLC shows that the reaction is complete, the temperature is lowered to 15-20°C, and the temperature is controlled at 15-20°C for 1 hour. Then it was filtered, and the filter cake was rinsed once with 13.82g of absolute ethanol to obtain the crude nitrendipine. The HPLC spectrum is shown in figure 1 . The result of HPLC area normalization method is that the chromatographic content of Ni...
Embodiment 2
[0048] Compared with Example 1, the only difference is that ethyl 3-nitrobenzylidene acetoacetate is prepared by reacting 3-nitrobenzaldehyde with ethyl acetoacetate and then recrystallizing from absolute ethanol. In this embodiment, refined 3-nitrobenzylidene ethyl acetate is used, and its purity is ≥99.9%.
[0049] A method for synthesizing high-purity Nitrendipine, using the following steps:
[0050] (1) Put 3-nitrobenzylidene acetoacetate and 3-aminobutenoic acid methyl ester in the organic solvent, the amount of 3-aminobutenoic acid methyl ester added is 3-nitrobenzylidene acetoacetate 100% of the molar amount of ethyl ester intermediate,
[0051] (2) Raise the temperature of the above-mentioned reactant to 70~75℃, then add a small amount of concentrated hydrochloric acid catalyst dropwise at this temperature. After the dropwise addition is completed, it will be controlled by TLC immediately. After the reaction has disappeared, the temperature will be lowered to crystallize , ...
Embodiment 3
[0054] Compared with Example 1, the only difference is that in step (2), after the temperature is raised to 70-75°C, there is no heat preservation reaction for 1 hour, and concentrated hydrochloric acid is directly added dropwise. The yield of the crude product was 46%, and the product purity and impurity control were similar to those of Example 1, but the yield was lower than that of Example 1.
[0055] Because there is no pre-heating and reaction for 1 hour, the yield is low, which affects the product yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com