A kind of fluorescent probe for detecting hydrogen persulfide and its synthesis method and application
A technology of hydrogen persulfide and fluorescent molecular probes, applied in chemical instruments and methods, fluorescence/phosphorescence, organic chemistry, etc., can solve the problems of excitation light interference sensitivity, insufficient, unfavorable detection of biological samples, etc., and achieve high sensitivity, optical Stable performance, high selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Synthesis of Fluorescent Molecular Probes
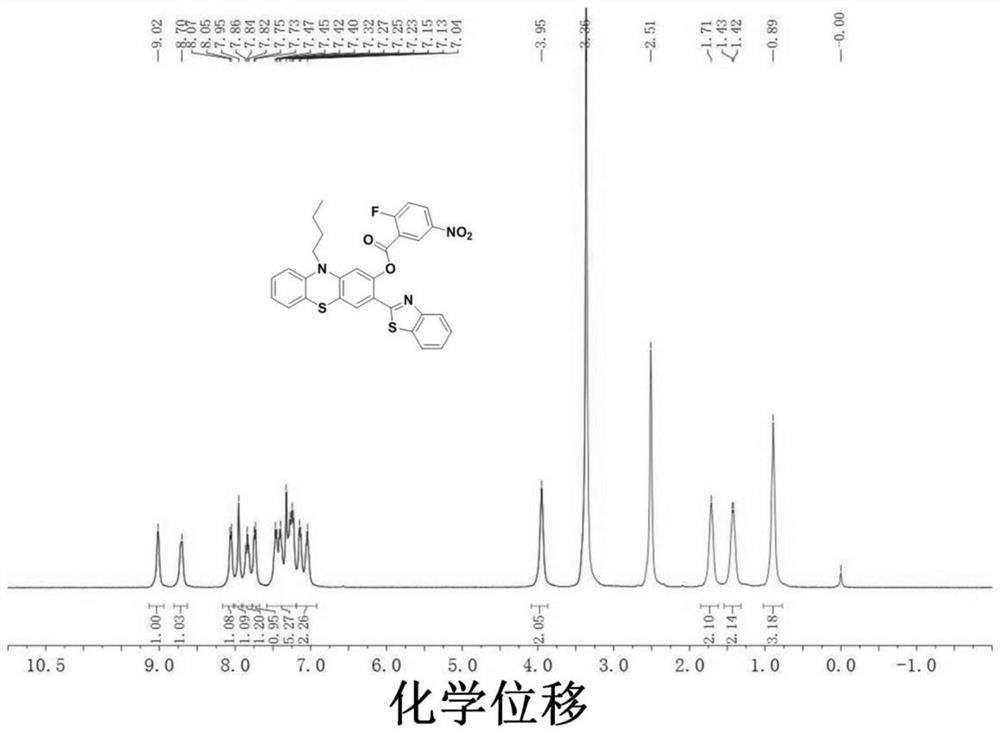
[0032] Compound 2 (80.8 mg, 0.2 mmol), 2-fluoro-5-nitrobenzoic acid (56 mg, 0.3 mmol), dicyclohexylcarbodiimide (54 mg, 0.26 mmol), 4-dimethylaminopyridine ( 4 mg, 0.034 mmol) was added to the solvent dichloromethane (15 mL) and reacted at room temperature for 12 h. After the reaction was completed, the solvent was distilled off under reduced pressure and separated by column chromatography (eluent: petroleum ether:ethyl acetate=5:1, v / v) to obtain 68.6 mg of solid product (yield: 87%). The product structural formula is as follows:
[0033]
[0034] 1H NMR(400MHz, DMSO)δ9.02(s,1H),8.70(s,1H),8.06(d,J=7.0Hz,1H),7.95(s,1H),7.91-7.77(m,1H) ,7.74(d,J=7.3Hz,1H),7.35(ddd,J=38.5,19.1,7.3Hz,5H),7.19–6.92(m,2H),3.95(s,2H),1.71(s,2H) ),1.42(d,J=5.7Hz,2H),0.89(s,3H).MS[ESI]:m / z,calcd for[M+H] + 572.1114, Found: 572.1121.
Embodiment 2
[0035] Example 2: Fluorescence detection of hydrogen persulfide by probe
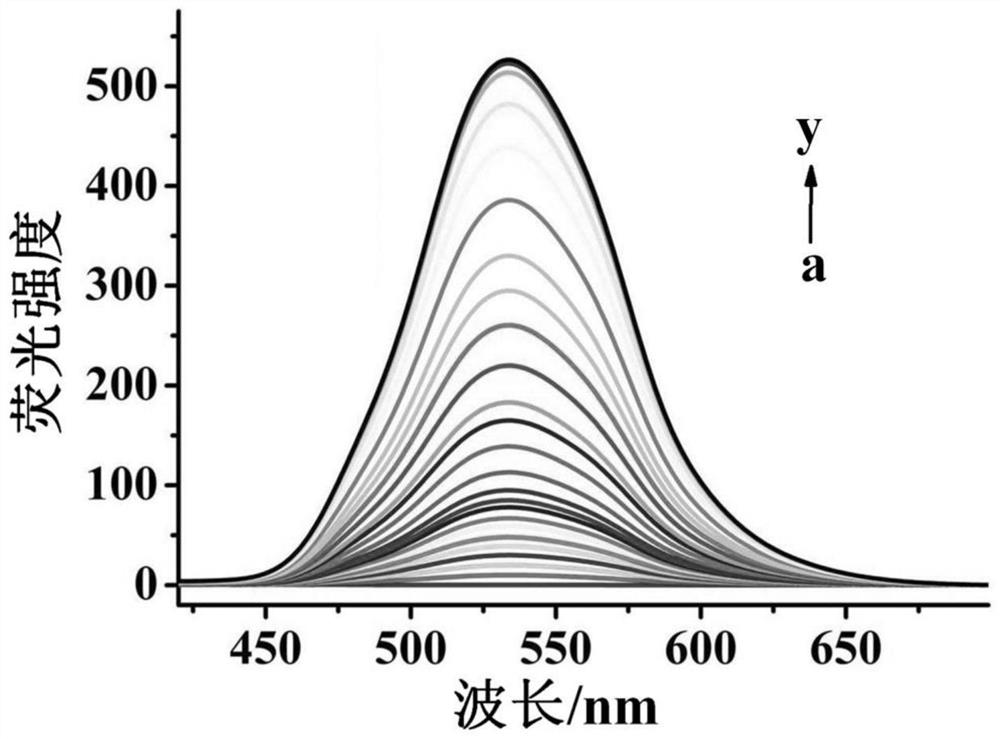
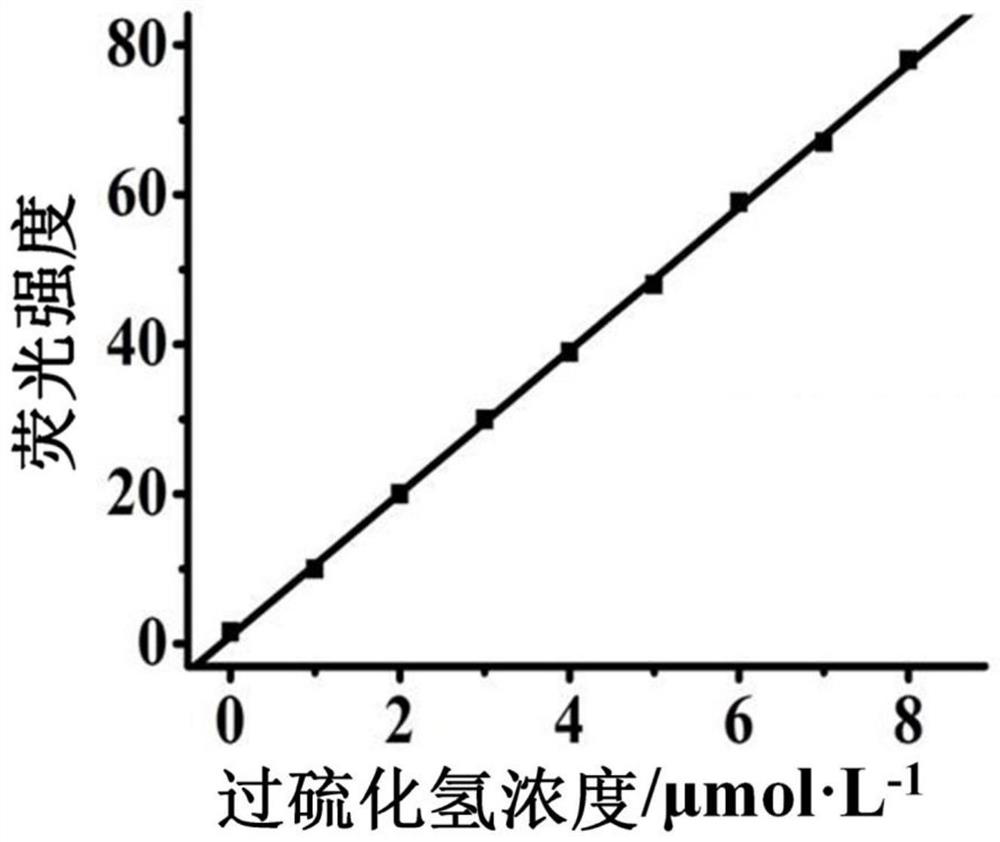
[0036] The molecular probe prepared above was dissolved in a mixed buffer solution of water and acetonitrile (H 2 O / CH 3 CN=1 / 1, v / v, 10mM HEPES, pH 7.4)), prepared as 5μmol·L -1probe solution. Add 2mL of prepared 5μmol·L to a 3mL cuvette -1 The probe solution of the present invention is then added with different concentrations of hydrogen persulfide and evenly mixed, and its fluorescence spectrum is tested, and the results are as follows figure 2 shown. The fluorescence emission intensity of the solution at 530nm was plotted against the concentration of hydrogen persulfide, and the concentration of hydrogen persulfide was 0–8 μmol·L -1 Within the range, there is a good linear relationship between the two ( image 3 ), can realize the quantitative detection of hydrogen persulfide in this concentration range. And this probe is not affected by some other common substances, such as S 2- , S 2 O ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com