Nitric oxide reductase analogs and preparation method thereof
A technology of nitric oxide and reductase, which is applied in the fields of chemical biology and biomimetic synthesis, can solve the problems of low content of natural NOR, and achieve good affinity, easy separation and characterization, and definite configuration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0059] The present invention will be described in further detail below in conjunction with specific examples, but the present invention is not limited thereto.
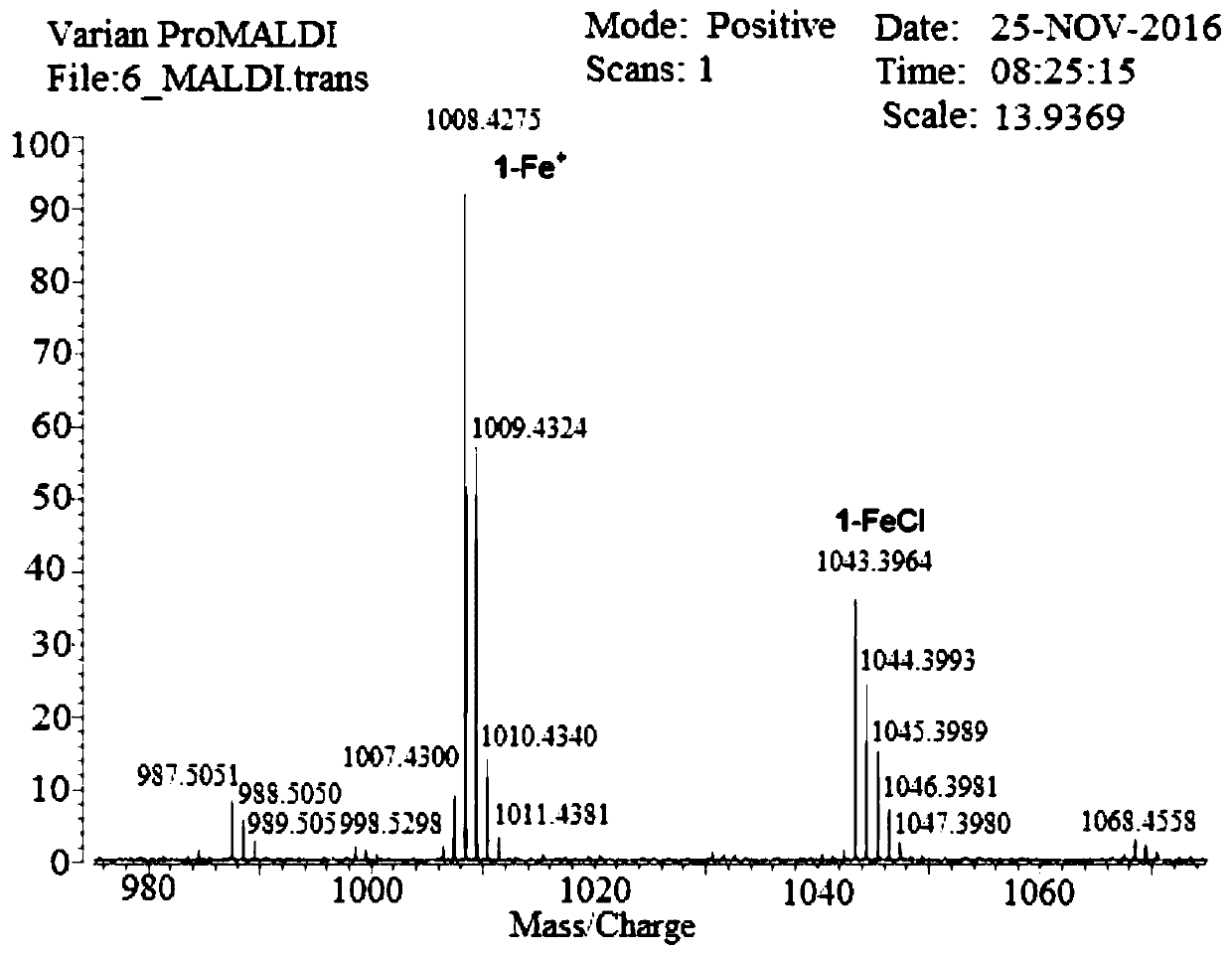
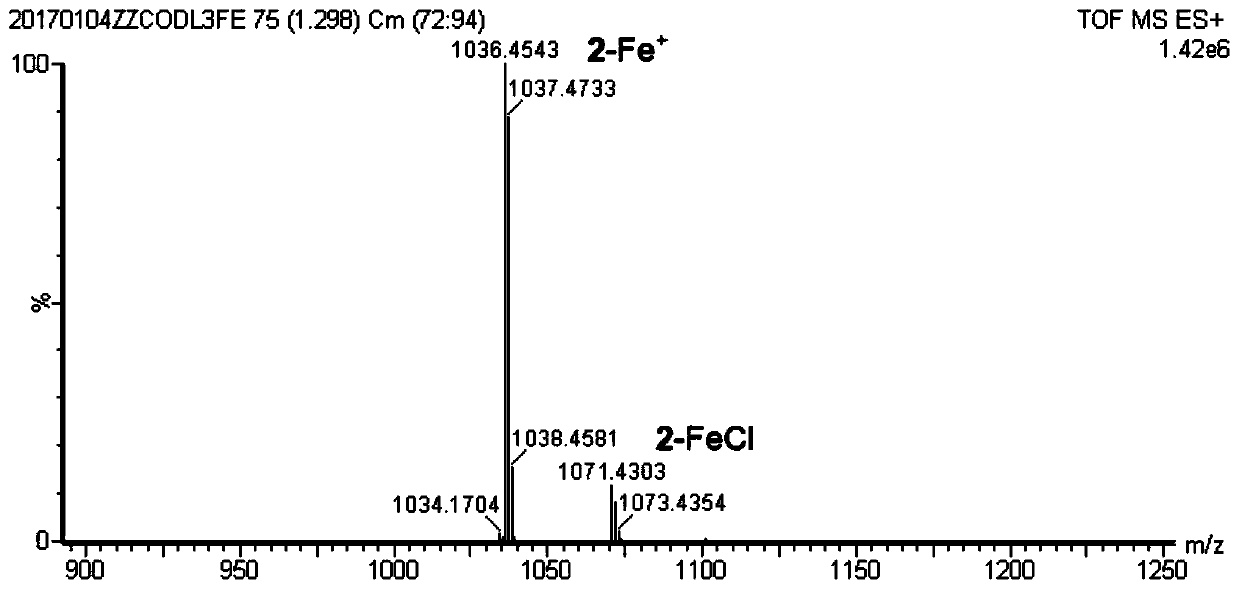
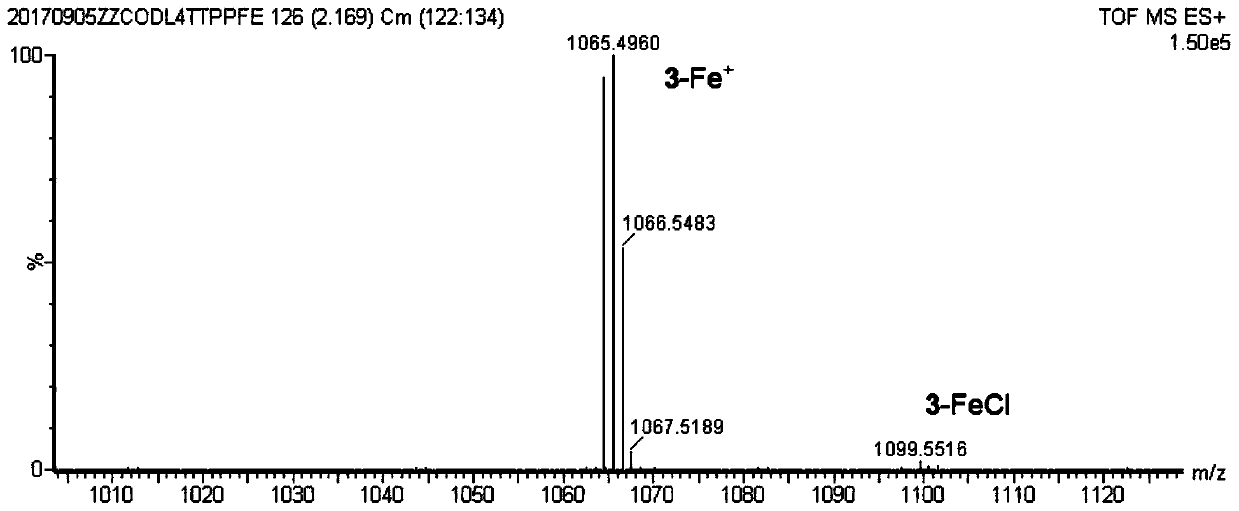
[0060] The following examples take the synthesis of iron porphyrin NO complex 2-FeNO as an example. The preparation, separation, characterization and structural characteristics of the series of iron porphyrin NO complexes are all described through this example, and the present invention is further illustrated by the accompanying drawings description of. Figure 1 to Figure 14 The mass spectrometry, ultraviolet-visible spectrometry, partial single crystal diffraction and other evidences of this series of complexes and the schematic diagram of the NO generation device are provided.
[0061] Iron porphyrin NO complex 2-FeNO and its precursor compound 2-FeCl, its compound synthesis route is as shown in formula (I):
[0062]
[0063] As can be seen from formula (I), it specifically comprises four steps:
[0064] The f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com