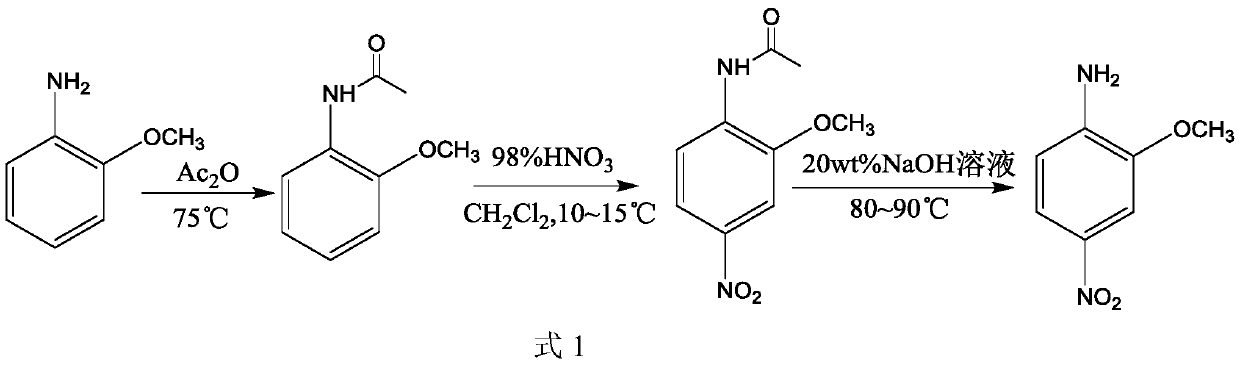
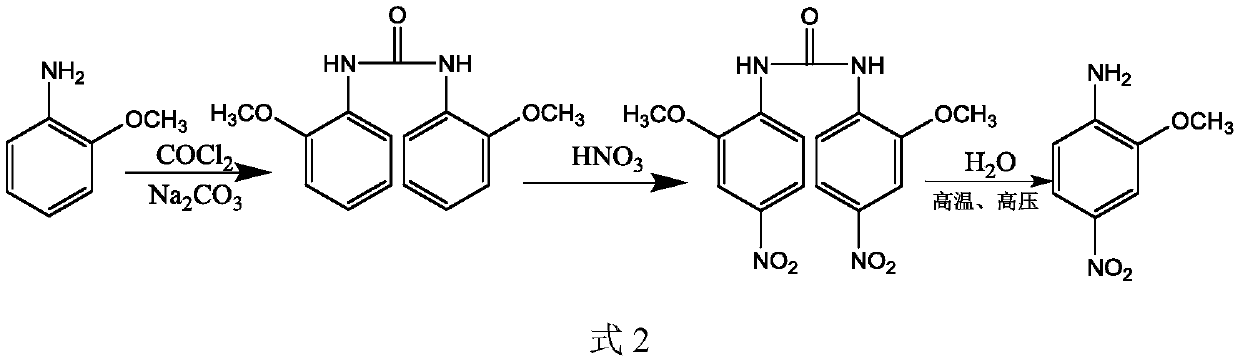
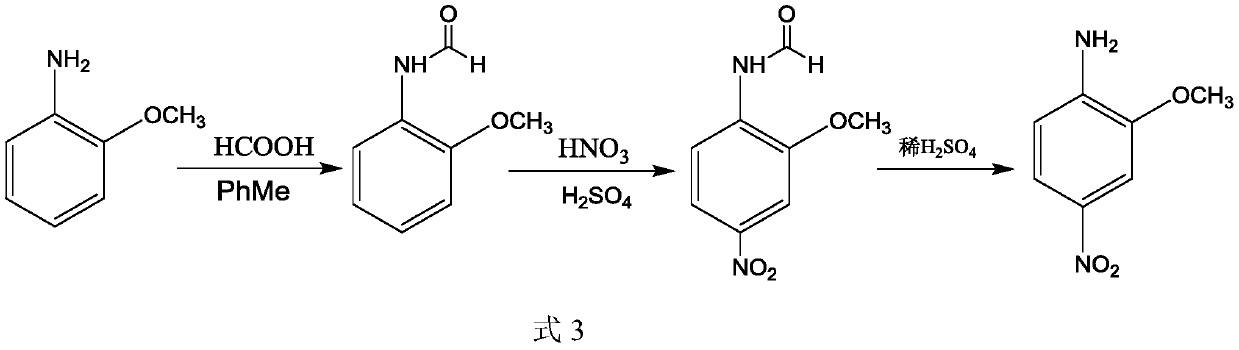
Preparation method of 2-methoxy-4-nitroaniline
A technology of o-methoxyaniline and nitroaniline, which is applied in the field of preparation of 2-methoxy-4-nitroaniline, can solve the problem of producing 2-methoxy-4-nitroaniline, which has not yet been industrialized. Unstable efficiency, high cost of raw materials, etc., to achieve good industrial application value, convenient product post-processing, and low cost of acylation reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Embodiment 1, a kind of preparation method of 2-methoxy-4-nitroaniline, carries out following steps successively:
[0052] 1) Take 123.2g (1.0mol) of 2-methoxyaniline and 150g (2.5mol) of acetic acid into a 500ml four-neck flask and raise the temperature to 115°C to start the reaction, and remove the water generated in the system by reactive distillation. After reacting for 6 hours, GC detected that the reaction of 2-methoxyaniline was completed, and the acetic acid solution of 2-methoxyacetanilide was obtained, weighing 233.7 g.
[0053] 2), the temperature of the reaction liquid (2-methoxyacetanilide in acetic acid solution) obtained in step 1) was lowered to 0-10°C, and then 77.2 g (1.2 mol) of fuming nitric acid (1.2 mol, 1 hour for dropping) was added dropwise thereto, and During the addition process, the temperature of the reaction system is controlled at 0-10°C (which can be realized by jacket cooling). Add 100 g of deionized water to the nitration reaction liqu...
Embodiment 2~ Embodiment 10
[0056] Keep steps 2) and 3) unchanged, change part of the reaction conditions in step 1) in Example 1, and the rest of the conditions are the same as in Example 1, to obtain Examples 2 to 10, respectively. The specific process parameters and the obtained results are shown in Table 1 below:
[0057] Table 1
[0058]
[0059]
Embodiment 11~ Embodiment 20
[0061] Keep steps 1) and 3) unchanged, change part of the reaction conditions in step 2) in Example 1, and the rest of the conditions are the same as in Example 1, to obtain Examples 11 to 20, respectively. The specific process parameters and the obtained results are shown in Table 2 below:
[0062] Table 2
[0063]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com