Simple synthesis method for sex pheromones of oriental fruit moths
The invention relates to a technology for the sex pheromone and synthesis method of the pear worm, which is applied to chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve the problems of long route, difficult access to raw materials, and inability to large-scale production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
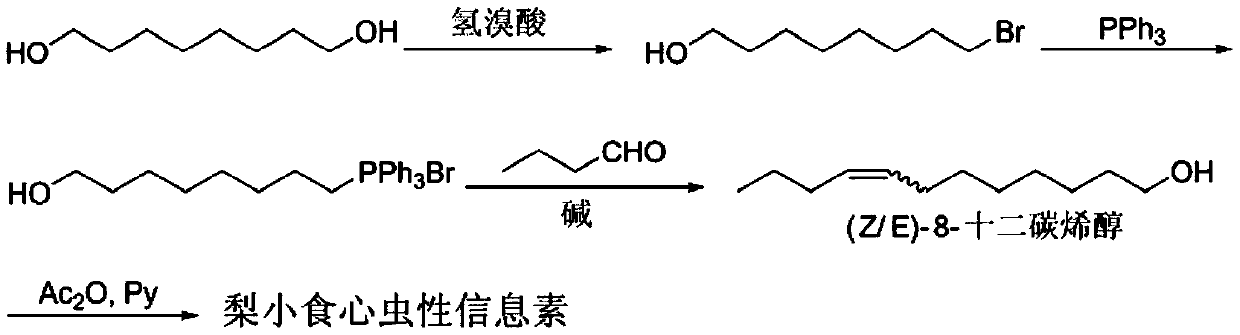
[0024] Synthesis of 8-bromooctanol
[0025] Add 1,8-octanediol (250g, 1.712mol), toluene (1000mL), 48% hydrobromic acid (231mL, 2.054mol, 1.2eq) into a 2L three-necked flask, heat to 110°C and reflux for 30 hours. Additional 48% hydrobromic acid (84mL, 0.753mol, 0.44eq) was added, heated to reflux for 20 hours, and a small amount of raw material remained as detected by GC. Cool to room temperature and dilute with 500mL petroleum ether, separate the hydrobromic acid, and wash the organic phase with saturated sodium bicarbonate (400mL×2) and saturated brine (400mL×2) successively, and dry over anhydrous sodium sulfate. Spin-dried to obtain 318g of 8-bromooctyl alcohol with a yield of 83%, which was directly cast into the next step.
Embodiment 2
[0027] Synthesis of 8-Hydroxyoctyltriphenylphosphine Salt
[0028] Add monobrominated product (318g, 1.424mol), acetonitrile (1000mL), triphenylphosphine (409g, 1.566mol, 1.1eq) into a 2L three-necked flask, heat to 94°C and reflux for 48 hours. After the reaction, cool to room temperature and spin dry acetonitrile. Add 500mL of toluene, heat to reflux until homogeneous and stir for 15min, cool to room temperature, and pour out the upper layer of toluene. Repeat the reflux twice. Spin-dry to obtain 602 g of viscous liquid, namely 8-hydroxyoctyl triphenylphosphine salt, with a yield of 88%.
Embodiment 3
[0030] Synthesis of (Z / E)-8-dodecen-1-ol
[0031] Add anhydrous DMSO (500mL) and 60% NaH (102g, 2.556mol, 2eq) into a dry 2L three-necked round-bottom flask, add a 1L storage ball to the middle mouth for buffering, and open the top of the storage ball upward to discharge hydrogen. Heat to 70°C and stir for 1 hour until there are no bubbles to obtain dimethyl sulfoxide sodium salt, and cool to room temperature under nitrogen protection for later use. Add 8-hydroxyoctyltriphenylphosphine salt (602g, 1.278mol) and 1000mL tetrahydrofuran into a 3L mechanically stirred three-neck flask, add the newly prepared sodium dimethyl sulfoxide dropwise, and stir at room temperature for half an hour. Cool to 0°C in an ice bath, add a solution of n-butyraldehyde (110g, 1.5336mol, 1.2eq) in tetrahydrofuran (200mL), dropwise complete, react at 0°C for half an hour, and stir at room temperature for 1 hour. Saturated ammonium chloride (500 mL) was added to quench, the organic phase was separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com