miRNA biosynthesis inhibitor
A compound, -COOR9 technology, applied in the field of chemical medicine, can solve the problems of drug clinical development failure, high cost, unclear understanding of protein signaling network, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1, the synthesis of compound of the present invention
[0062]
[0063] Ethyl 2-(4-methoxyphenyl)-4-methylthiazole-5-carboxylate (T2): Add 4-methoxybenzamide (152.2mg, 1mmol) in a 25mL round bottom flask, Lawson's reagent (485.4mg, 1.2 mmol) and THF (20 mL). After reacting for 4 hours at room temperature, Lawson's reagent and solvent were removed, and the intermediate thiobenzamide was separated and purified by column chromatography, and then mixed with ethyl 3-bromo-2-oxobutanoate (209 mg, 1 mmol) in ethanol (10 mL) was used as a solvent, refluxed at 70°C for 4 h under reaction conditions, after the reaction was completed, the solvent was removed, and compound T2 was obtained by separation and purification by column chromatography. Yield 92%, white solid, developing system PE / EA 10:1 (Rf=0.5, PE / EA=3:1), 1 H NMR (400MHz, CDCl 3 )7.91(d, J=8.8Hz, 2H), 6.96(d, J=8.8Hz, 2H), 4.37(q, J=7.1Hz, 2H), 3.86(s, 3H), 2.76(s, 3H) ,1.68(s,1H),1.40(t,J=7.1Hz,3H); ...
experiment example 1
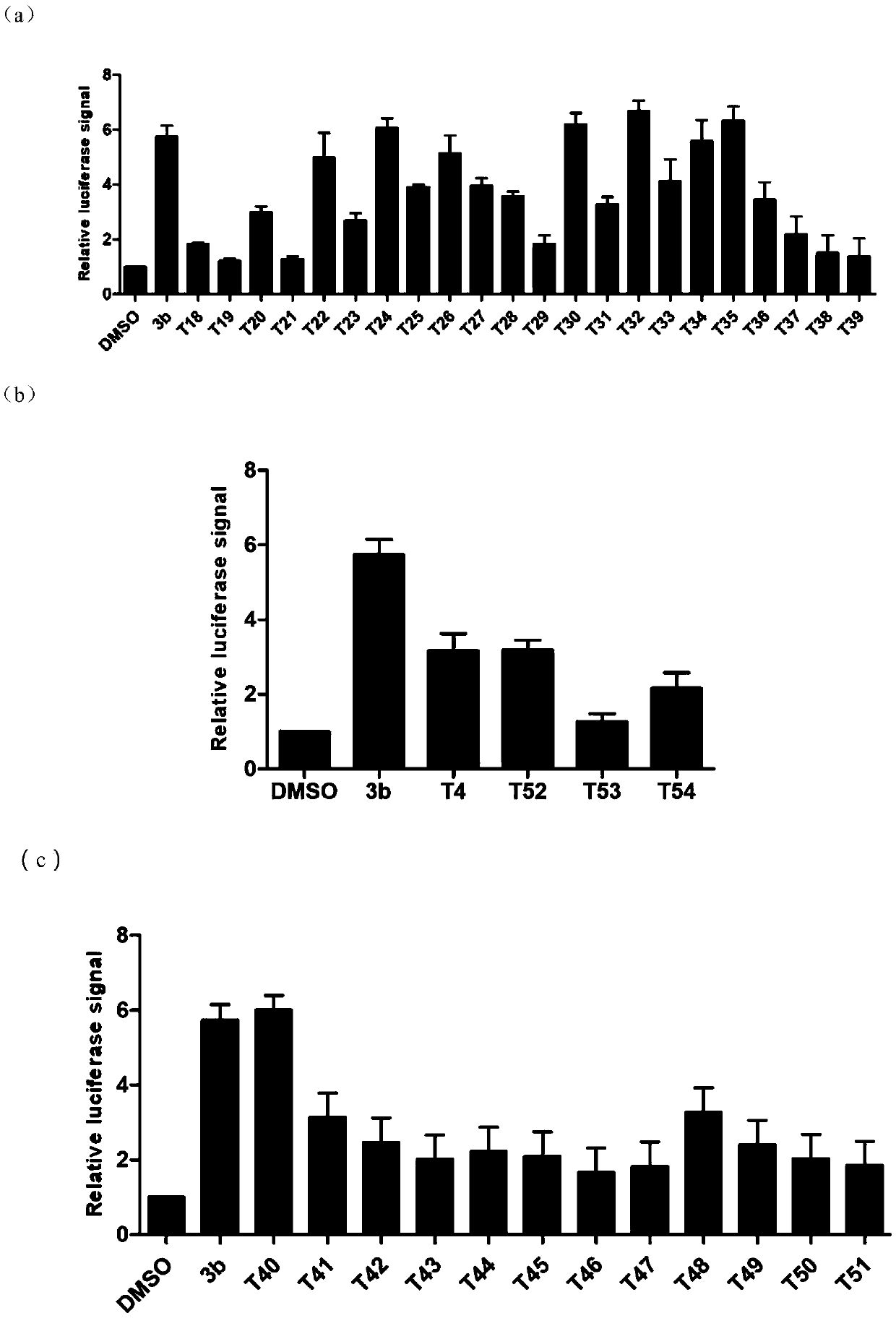
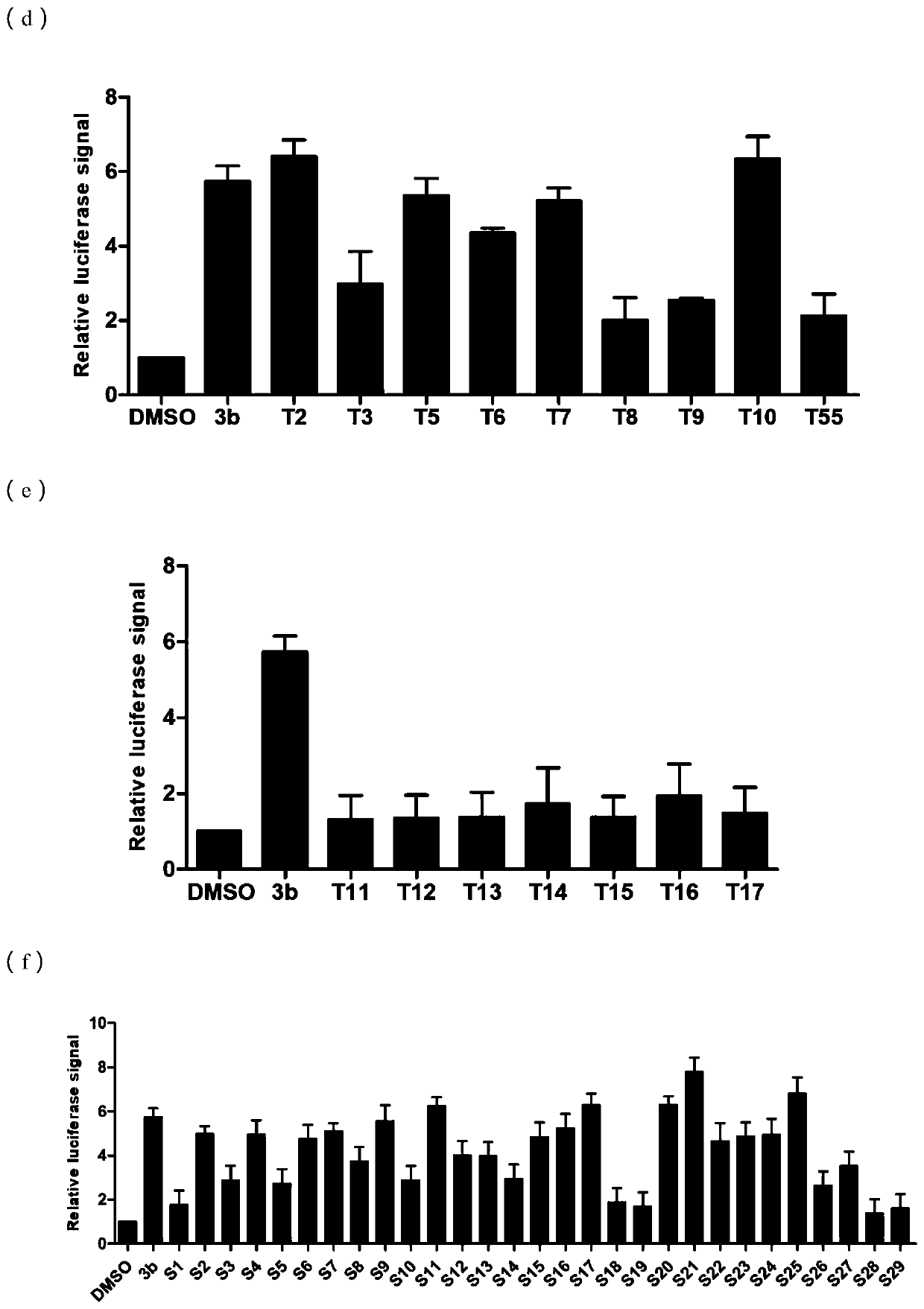
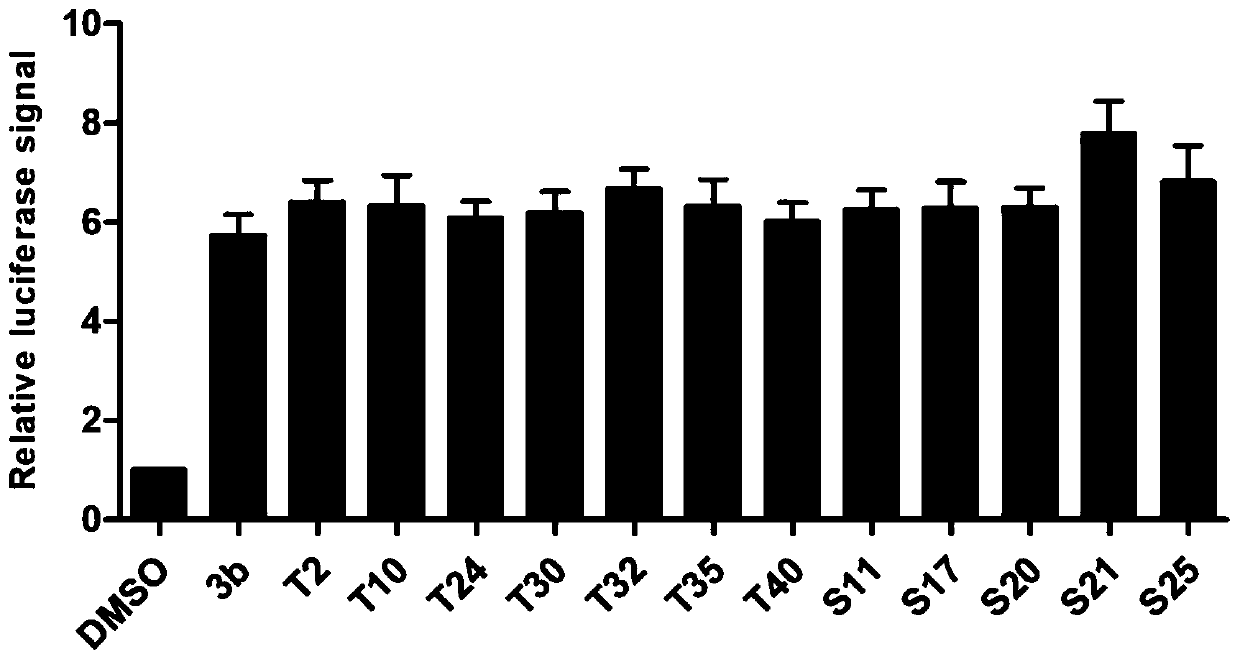
[0238] Experimental Example 1. Analysis of the Inhibitory Activity of Compounds on miRNA-21
[0239] 1. Experimental method:
[0240] (1) Experimental materials and instruments
[0241] a. Main reagents: DMEM / high glucose medium, PBS buffer, EDTA-0.25% trypsin, penicillin streptomycin (double antibody), BIOMYC-3Antibiotic Solution, etc. were purchased from Hyclone Company; fetal bovine serum was purchased from Gibco Company; Coenzyme A sodium salt hydrate and D-Luciferin sodium salt were purchased from Sigma; DMSO and G418 were purchased from Amresco.
[0242] b. Main instrument: CO 2 Constant temperature cell incubator, ultra-clean workbench, full-wavelength scanning multi-function reader, cell operation table, optical inverted microscope, pressure steam sterilizer, micro centrifuge, electronic constant temperature water bath, horizontal shaker, etc.
[0243] (2) Establishment of cell model
[0244] Construction of a cell screening model for small molecule inhibitors of m...
experiment example 2
[0259] Experimental example 2. Affinity analysis of compound docking with TRBP-related binding protein in the process of miRNA biosynthesis 1. Experimental method:
[0260] In our previous study, we combined 7 reported TRBP-related binding proteins in miRNA biosynthesis (from the PDB database, 4 from mammalian and 2 from Drosophila genes and 1 from plant) Molecular docking was carried out with compound 3b, and it was found that only two protein crystal models from mammals (4WYQ: Dicer-TRBP and 3LLH: TRBP (dsRBD2)) could form an active pocket with 3b.
[0261] TRBP acts as a Dicer partner in animal cells. TRBP can affect the malignant metastasis of cancer cells by affecting the miRNA maturation mediated by Dicer and Ago2. TRBP has been proved to be indispensable in the process of RNA interference (RNAi); TRBP and Dicer Or Ago2 interacts and combines with dsRNA to form a RISC complex (RNA induced silencing complex) to perform gene silencing function, which can process pre-miRNA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com