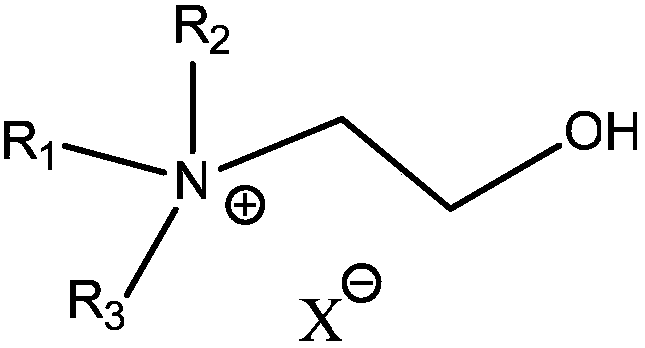
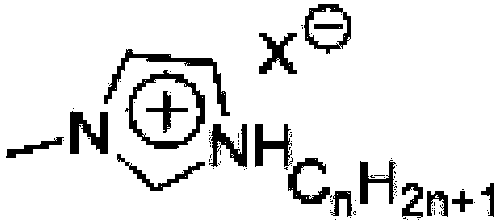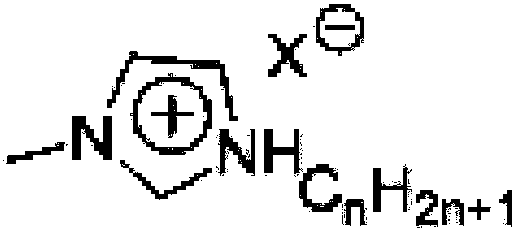Method for high-efficiency synthesizing of 5-hydroxymethyl furfural by adding nitrogen-containing compound
A technology of hydroxymethylfurfural and nitrogen compounds, which is applied in the field of high-efficiency synthesis of 5-hydroxymethylfurfural, can solve the problems of loss in the separation process, expensive organic ligands, and affecting the recovery purity of 5-hydroxymethylfurfural, so as to improve the selection performance, optimize the effect of catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 500mg of [BMIM]Cl, 400mg of D-glucose, 52.9mg of CrCl3-6H2O and 100ul of deionized water into a 4ml small reaction bottle, seal the reactor and carry out the reaction. The reaction temperature is 100°C. The stirring speed is 300rpm, the reaction time is 1h, and the reaction is quenched with cold water after the reaction is completed.
[0032] Preheat 500mg of ionic liquid butyltrimethylimidazolium chloride ([BMIM]Cl) to 100°C, add it into a 4ml reaction flask, then add 100ul deionized water, 52.9mg of CrCl 3 -6H 2 O. After the mixture in the bottle was cooled to room temperature, 400 mg of D-glucose was added, and then the reactor was sealed and reacted at 100° C. for 1 hour, and the stirring speed was 300 rpm during the reaction. After the reaction was completed, the reaction was quenched with cold water. Reactants and products were analyzed by high performance liquid chromatography. The results are shown in Table 1.
Embodiment 2
[0034] Preheat 500mg of ionic liquid [BMIM]Cl to 100°C, add it into a 4ml reaction flask, then add 100ul deionized water, 52.9mg of CrCl 3 -6H 2 O, add tetramethylethylenediamine with the same amount as the Cr substance, after the mixture in the bottle is cooled to room temperature, add 400 mg of D-glucose, then seal the reactor and react at 100°C for 1 hour, stirring during the reaction The rotational speed is 300rpm. After the reaction was completed, the reaction was quenched with cold water. Reactants and products were analyzed by high performance liquid chromatography. The results are shown in Table 1.
Embodiment 3
[0036] Preheat 500mg of ionic liquid [BMIM]Cl to 100°C, add it into a 4ml reaction flask, then add 100ul deionized water, 52.9mg of CrCl 3 -6H 2O, add s-triazine with the same amount as the Cr substance, after the mixture in the bottle is cooled to room temperature, add 400 mg of D-glucose, then seal the reactor and react at 100°C for 1 hour, and the stirring speed during the reaction is 300rpm . After the reaction was completed, the reaction was quenched with cold water. Reactants and products were analyzed by high performance liquid chromatography. The results are shown in Table 1.
[0037] The effect of different organic amine additives in the chromium chloride system in table 1
[0038]
[0039] Note: 400mg Glucose, 500mg [BMIM]Cl, CrCl 3 .6H 2 The molar ratio of O to glucose=10:100; additives and CrCl 3 .6H 2 The molar ratio of O=1:1; 100℃, react for 1h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com