Artificial antigen presenting cell applied to efficiently amplifying NK and construction method thereof
A technology of NK cells and artificial antigens, applied in the biological field, can solve problems such as expansion or activation of NK cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0203] In a specific embodiment, the present invention provides a method for efficiently preparing NK cells, comprising the following steps:
[0204] 1) The tandem gene IL-15-IL-21-MICA was transfected into K562 cells using a lentiviral transfection system for gene expression screening, and the cells with successful gene expression were amplified and irradiated at a dose of 200Gy. The irradiated K562 cells were then used as feeder cells for preparing NK cells;
[0205]2) After resuspending the PBMCs isolated from peripheral blood in culture medium, they were mixed with the above-mentioned modified irradiated K562 at a ratio of 1:1 for culture, and IL with a final concentration of 1000IU / mL was added to the culture medium -2 was cultured and recorded as day 0.
[0206] 3) On day 7, add inactivated K562 cells again for secondary stimulation;
[0207] 4) Continue to expand the culture until the 14th day, harvest the cells, and complete the preparation of NK cells.
[0208] As ...
Embodiment 1
[0221] 1. The construction steps of feeder cells are as follows:
[0222] (1) Vector construction
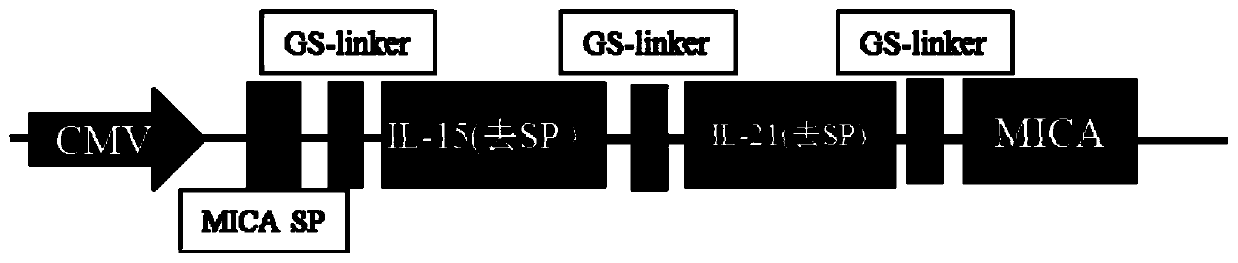
[0223] The tandem gene Il-15-IL-21-MICA nucleotide fragment (SEQ ID NO.: 1) synthesized by the gene was connected to the pCDH-CMV-MCS-EF1-Puro vector (such as figure 1 shown). Transform Stbl3 Escherichia coli strain with the vector, screen with ampicillin, obtain positive clones, extract plasmids, identify the clones by enzyme digestion, and obtain the target vector.
[0224] (2) Lentivirus preparation
[0225] Based on the lentivirus packaging scheme of Lipofectamine2000 transfection reagent and pLP1, pLP2, pLP / VSVG, pLVX-shRNA four-plasmid system, the prepared 293FT (human embryonic kidney cells) cells of P10-P12 were used for lentivirus by instant transfection method For packaging, the molar ratio of pLP1, pLP2, and pLP / VSVG is 1:2:1, the amount of the four plasmids is about 20ug, and the concentration of each plasmid is >0.5ug / ul. A 10cm cell culture plate was used for t...
Embodiment 2
[0235] For better illustration, an experimental group and a control group are set up in the embodiment. The control group used K562 without gene modification, and the other culture conditions and treatment conditions were the same. The two groups of cells were observed and detected.
[0236] The test results are as follows:
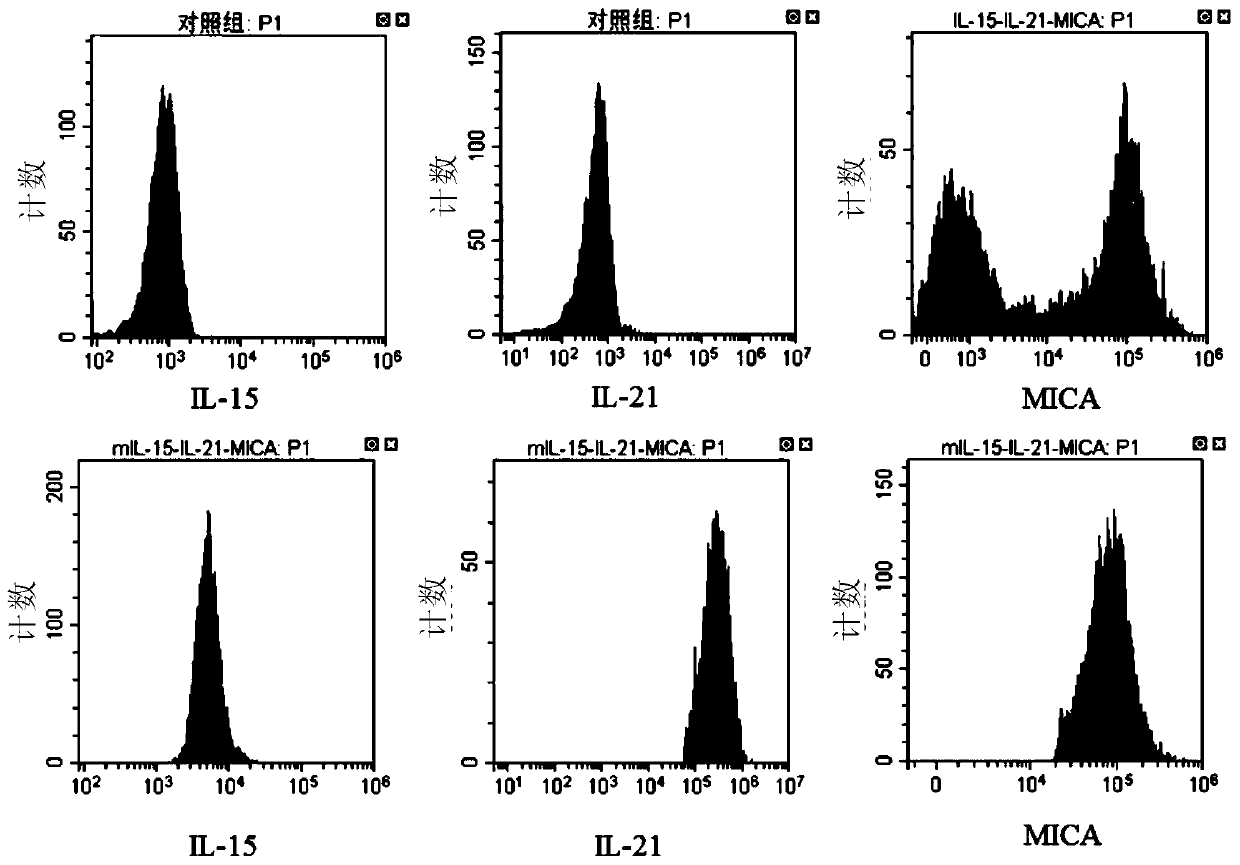
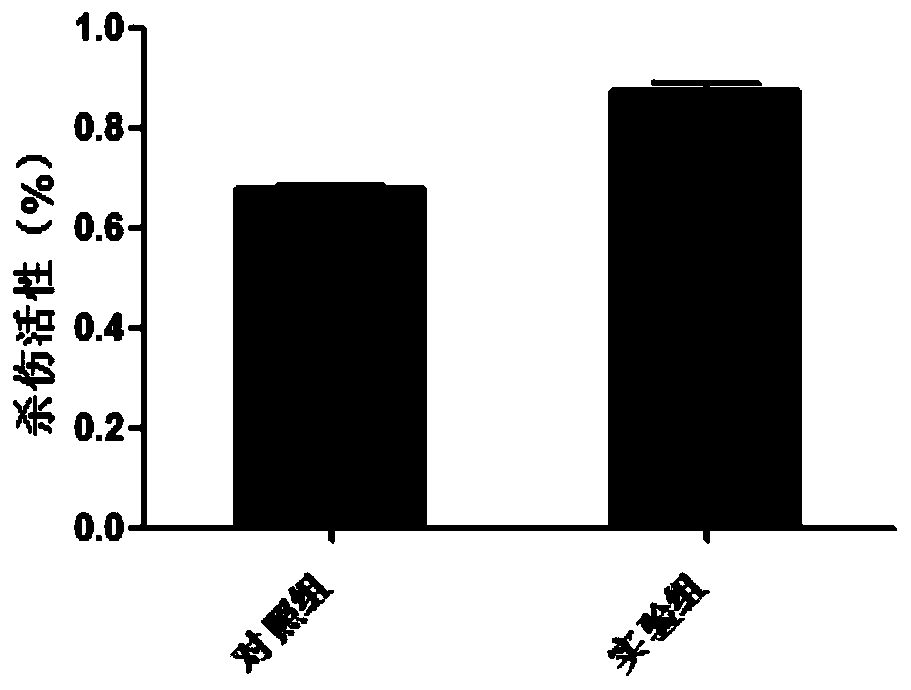
[0237] 1. Count the cells in the experimental group and the control group on the 0th, 14th, and 21st days of culture. At the same time, on the 14th and 21st days of cell culture, the cells cultured in the experimental group and the control group were analyzed and detected by cell immunophenotype. The CD3 and CD56 flow cytometry antibodies were used to incubate with the cells of the experimental group and the control group washed in PBS, and the incubation conditions were: 4°C, 30min. Then wash twice with PBS. The treated cells were tested on the machine. The results are shown in Table 1 and figure 2 Shown: the cell expansion multiple of the experim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com