Nucleotide sequence encoding CAR (Chimeric Antigen Receptor), ROBO1 CAR-NK cell expressing CAR as well as preparation and application of ROBO1 CAR-NK cell
A nucleotide sequence and encoding technology, applied in the field of ROBO1CAR-NK cells, can solve the problems of increasing the difficulty of clinical risk operation, endangering the life of patients, on-target/off-target toxicity and neurotoxicity, etc., so as to improve anti-tumor performance and effectively kill tumors , improve the effect of targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The preparation of embodiment 1 lentiviral vector
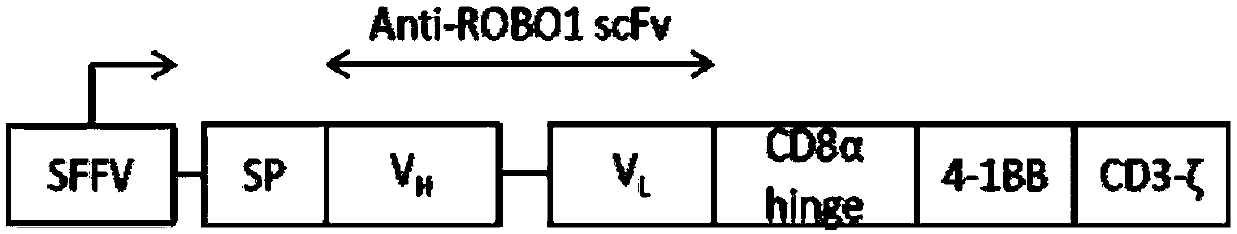
[0104] SCFV (Anti ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene sequence (its amino acid sequence is shown in SEQ ID NO: 1, gene sequence is shown in SEQ ID NO: 2) and mutant SCFV (Anti ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene sequence were synthesized respectively ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene sequence (the amino acid sequence is shown in SEQ ID NO: 3, and the gene sequence is shown in SEQ ID NO: 4). Taking SCFV(Anti ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene as an example to illustrate the preparation process of ROBO1 CAR-NK cells, preparation of mutant SCFV(Anti ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene The process of ROBO1M CAR-NK cells is the same.
[0105] Through enzyme digestion, the SCFV (Anti ROBO1-FN3)-CD8-4-1BB-CD3ζ fusion gene sequence was transformed and ligated into the PRRSLIN vector, and the upstream of the gene was the EP-1α promoter. Transform the Stbl3 E. coli strain with the vector, screen with ampicillin, o...
Embodiment 2
[0106] The preparation of embodiment 2 lentiviruses
[0107] (1) 24 hours before transfection, use about 8×10 per dish 6 293T cells were seeded into 15cm culture dishes. Make sure that the 293T cells are at about 80% confluence and evenly distributed in the culture dish during transfection.
[0108] (2) Prepare solution A and solution B
[0109] Solution A: 6.25ml 2×HEPES buffer (the amount packed in 5 large dishes, the effect is the best).
[0110]Solution B is a mixture obtained by adding the following plasmids: 112.5 μg PRRLSIN-SCFV (anti ROBO1-FN3) (target plasma); 39.5 μg pMD2.G (VSV-G envelope); 73 μg pCMVR8.74 (gag, pol, tat, rev); 625 μl of 2M calcium ion solution. Total volume of solution A: 6.25ml.
[0111] Mix solution B well, and while vortexing solution A gently, add solution B drop by drop to obtain a mixed solution of A and B, and let it stand for 5-15 minutes. Gently vortex the above mixed solution of A and B, add drop by drop to the culture dish containi...
Embodiment 3
[0112] Example 3 Preparation of ROBO1 CAR-NK cells
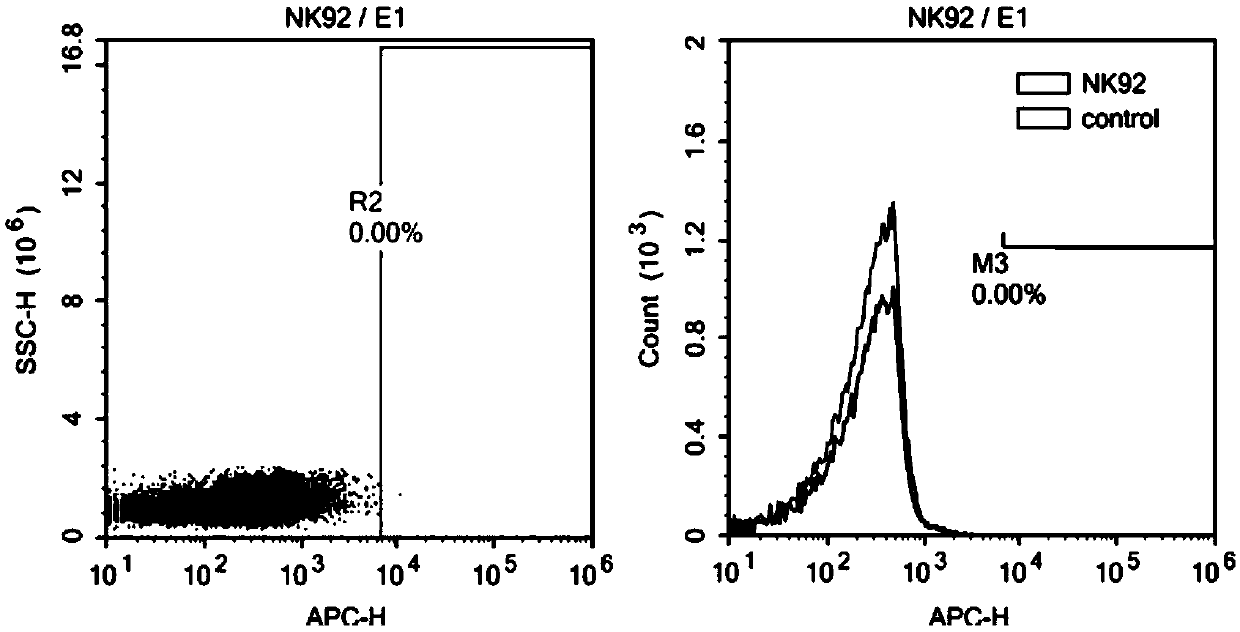
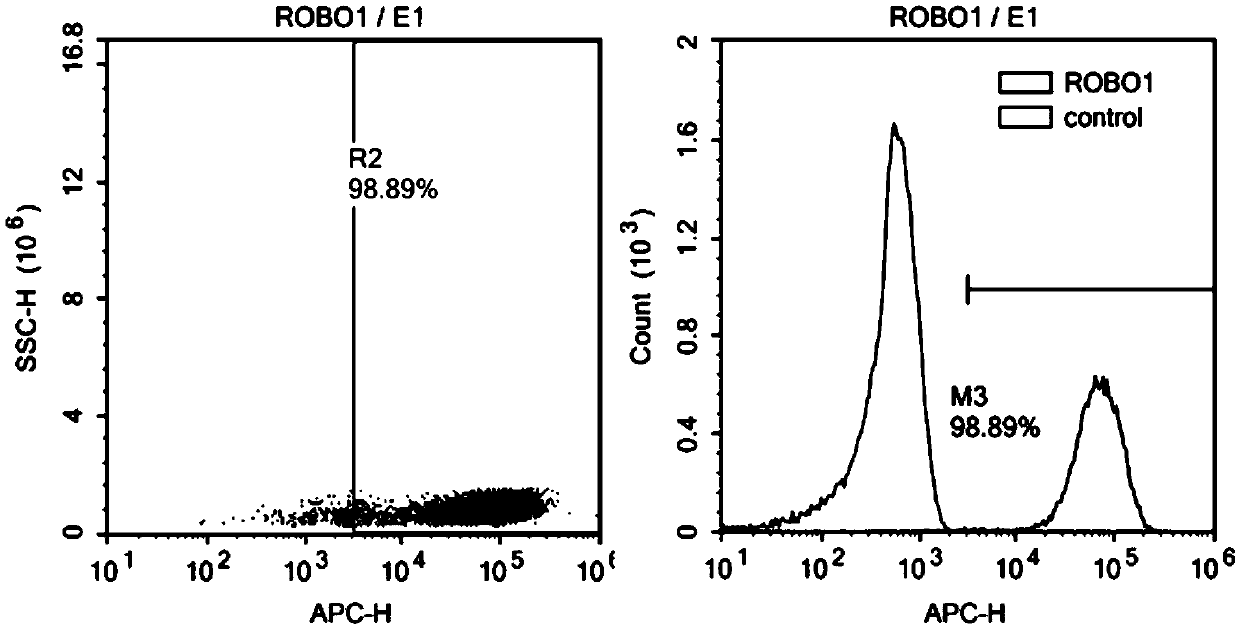
[0113] Adjust the NK-92 cell density to 2-3 x 10 5 / ml, according to the volume ratio (V / V) virus: cell culture medium = 1:5, the virus prepared in Example 2 was added, and polybrene 8 μg / ml was added at the same time. After 4 h, add an equal amount of fresh complete medium to adjust the cell density to 1×10 5 / ml to continue the culture. The next day, all the cells were centrifuged, fresh medium was added, and the culture was continued. Rehydrate every 1-2 days to maintain the cell density at 2-3×10 5 / ml. After 72 hours, CAR antibody staining was performed, and at the same time, ROBO1 CAR NK-92 positive cells were sorted by flow cytometry and expanded for culture. Observe the color change of the medium, cell density, and cell shape every day and make corresponding records.
[0114] After flow sorting, the positive ROBO1 CAR NK-92 cells were continuously cultured in the GMP workshop, expanded to the volume required fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com