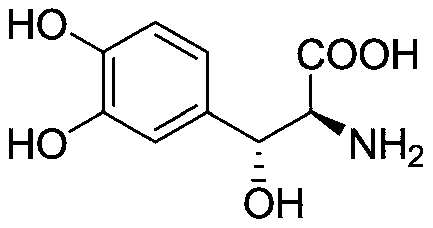
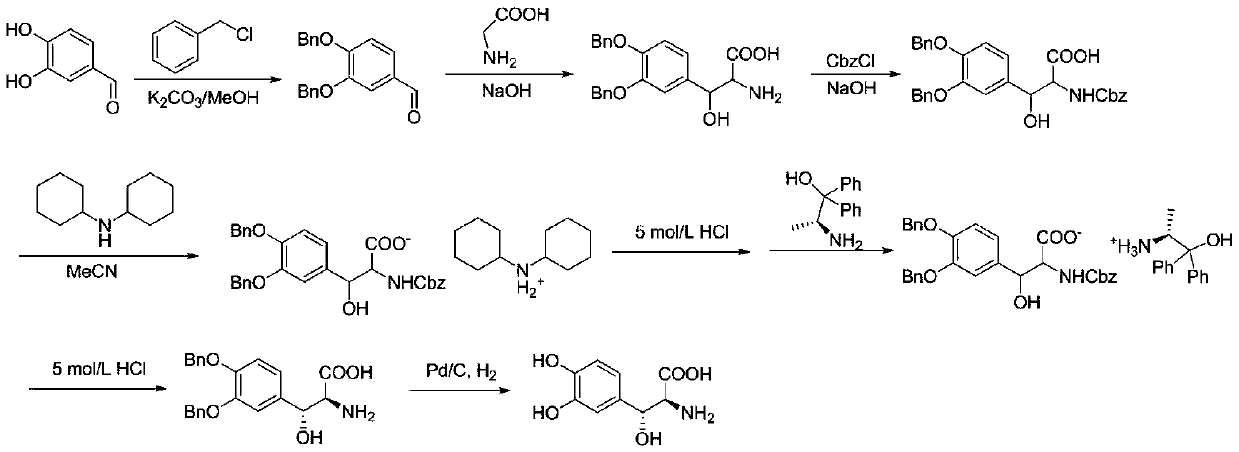
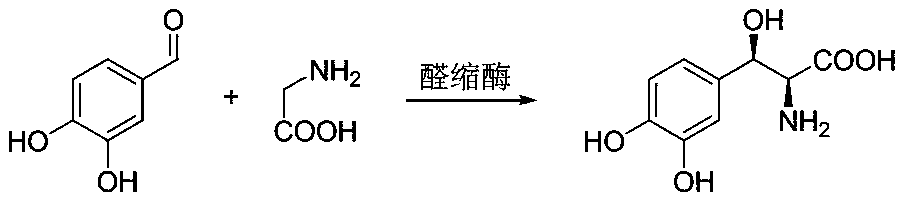
Method for preparing droxidopa
A technology of coenzyme and enzyme reaction, applied in the field of preparation of droxidopa, can solve the problems of large amount of glycine, poor stereoselectivity, and lack of conditions for industrialization, etc., and achieve the effect of simple operation, mild conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 10g 3,4-dihydroxybenzaldehyde, 100mg sodium bisulfite, 10g L-threonine, 40mg pyridoxal phosphate, 300mL 100mM phosphate buffer (pH7.5 ) heated to 30° C. and stirred evenly by magnetic force, adding 1 g of transaldolase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd., product number is YH1065, only one of the models is given here to illustrate the effect of the present invention), Stirring reaction was started, and after 20 hours, a sample was taken by HPLC to detect that the conversion rate was above 80%. After the reaction is finished, adjust the pH of the system to 1 with hydrochloric acid, heat up to 80°C for 2 hours, filter, and remove the protein. The filtrate was concentrated to dryness, and the residue was dissolved in isopropanol. The insoluble matter was removed by filtration, the pH was adjusted to about 6 with triethylamine, and a solid was precipitated to obtain 8.2 g of the product, ee=94%, dr=96:4.
Embodiment 2
[0035] Add 100g of 100mM phosphate buffer (pH=7.5) to a 250mL reaction flask under argon atmosphere, add 100mg of sodium bisulfite, 10g of 3,4-dihydroxybenzaldehyde, 10g of L-threonine and glucose under stirring 12 g, the system was stirred for 10 minutes. Use 3M sodium hydroxide to adjust the pH of the system to 7.5, control the temperature of the system to 35°C and stir evenly, then add 20 mg of pyridoxal phosphate, 20 mg of nicotinamide adenine dinucleotide, and 0.5 g of transaldolase enzyme powder (purchased from Suzhou Yinhang Biotechnology Co., Ltd., product number is YH1065), 0.5g acetaldehyde reductase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd., product number is YH2012) and 0.5g glucose dehydrogenase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd. (the product number is YH1901), started the stirring reaction, and kept the pH of the system at 7.5 with 3M NaOH during the reaction. After 20 hours, a sample was taken for HPLC det...
Embodiment 3
[0037] Add 100g of 100mM phosphate buffer (pH=7.5) to a 250mL reaction flask under argon atmosphere, add 100mg of sodium bisulfite, 10g of 3,4-dihydroxybenzaldehyde, 10g of L-threonine and 10g of isopropanol, and the system was stirred for 10 minutes. Use 3M sodium hydroxide to adjust the pH of the system to 7.5, control the temperature of the system to 35°C and stir evenly, then add 20 mg of pyridoxal phosphate, 20 mg of nicotinamide adenine dinucleotide, and 0.5 g of transaldolase enzyme powder (purchased from Suzhou Yinhang Biotechnology Co., Ltd., product number is YH1065), 0.5g acetaldehyde reductase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd., product number is YH2012) and 0.5g alcohol dehydrogenase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd., product number is YH2023), start stirring reaction, after 20 hours sampling HPLC detection, the conversion rate is more than 99%. After the reaction is finished, adjust the pH of the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com