Test strip for testing residual spiramycin
A technology of spiramycin and test strips, which is applied in the field of colloidal gold enzyme-linked immunosorbent rapid detection, can solve the problems of residue, consumer and environmental impact, and achieve the effect of low cost, simple storage and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Preparation of spiramycin detection test paper card
[0042] 1. Hapten synthesis
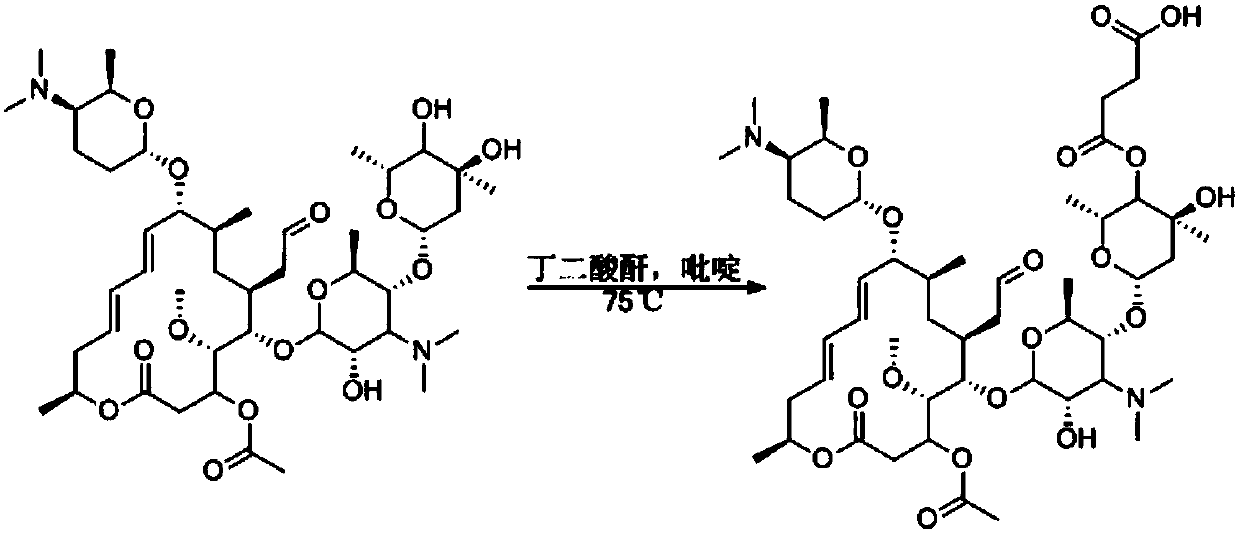
[0043] Weigh 5g of spiramycin and 6-8g of succinic anhydride.
[0044] (1) Dissolve 5g of spiramycin in an appropriate amount of pyridine, add 6-8g of succinic anhydride, and react at 75°C for 6-12h;
[0045] (2) TLC thin-layer plate detection reaction, until there is no raw material or the raw material point is very shallow, stop the reaction;
[0046] (3) Silica gel column purification and concentration to obtain the product, which is a hapten, and 2.3 g of the product was obtained, with a yield of 46%. The reaction technical route is as follows:
[0047]
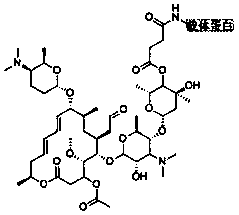
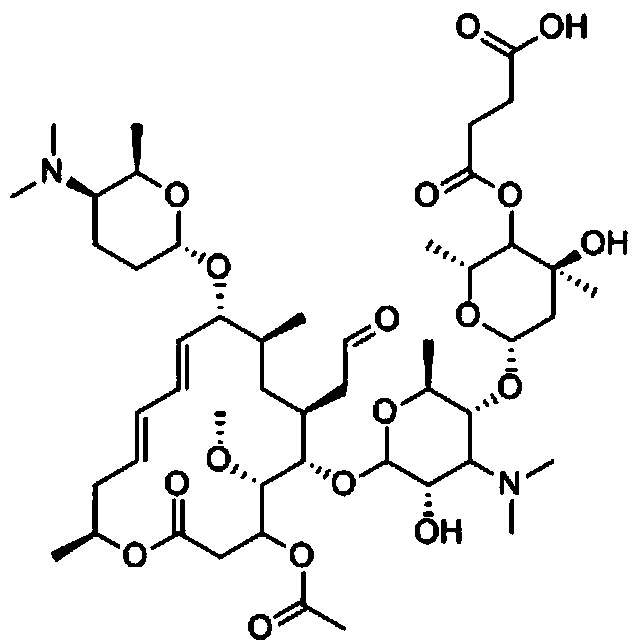
[0048] The molecular structural formula of the hapten obtained by the technical route is shown in Formula I:
[0049] (Formula I).
[0050] 2. Synthesis and identification of spiramycin hapten-carrier protein conjugates
[0051] (1) Synthesis of spiramycin hapten-ovalbumin conjugate
[0052]35 mg of spiramycin ha...
Embodiment 2
[0076] Example 2 Detection of Spiramycin Residues in Samples
[0077] 1. Sample pretreatment
[0078] Weigh 2.0±0.05g of homogenized tissue and feed samples into a centrifuge tube, add 1mL of deionized water, and tightly cap the bottle. Shake for 5 minutes, draw more than three drops of the solution and pour it into a 1.5mL centrifuge tube. If there is obvious yellow turbidity, please centrifuge, and then use the supernatant as the sample solution to be tested.
[0079] 2, detect with the test paper card of the present invention
[0080] Use a dropper to drop 3 drops of sample into the hole of the reagent card, and observe the result after 5-8 minutes. If it exceeds 10 minutes, the test result of the sample will be invalid.
[0081] 3. Analysis of test results
[0082] When the concentration of spiramycin in the sample is higher than 5ng / mL, the colloidal gold antibody will fully bind to spiramycin, so that no red band will appear in the (T) area because the competition r...
Embodiment 3
[0083] Example 3 Example of sample detection
[0084] Take 20 samples each of pork, chicken, premix and batch materials and 20 samples of various negative samples with known spiramycin residue concentration greater than 5ng / g, and calculate the negative and positive rates. The results are shown in Table 1-Table 4.
[0085] batch
Positive Pork Samples (20)
Negative Pork Samples (20)
1
20 positives
20 negative
2
20 positives
20 negative
3
20 positives
20 negative
[0086] batch
Positive Chicken Samples (20)
Negative Chicken Samples (20)
1
20 positives
19 negative, 1 positive
2
20 positives
20 negative
3
20 positives
19 negative, 1 positive
[0087] batch
Positive premix samples (20 parts)
Negative premix samples (20 parts)
1
20 positives
20 negative
2
20 positives
19 negative, 1 positive
3
20 posit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com