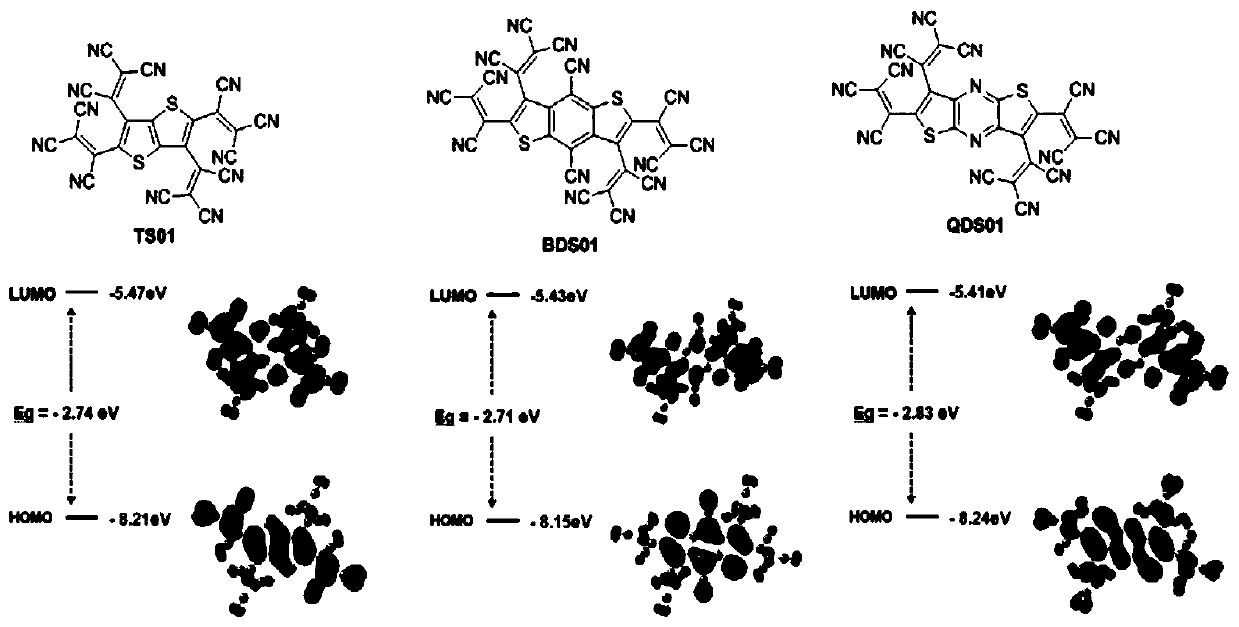
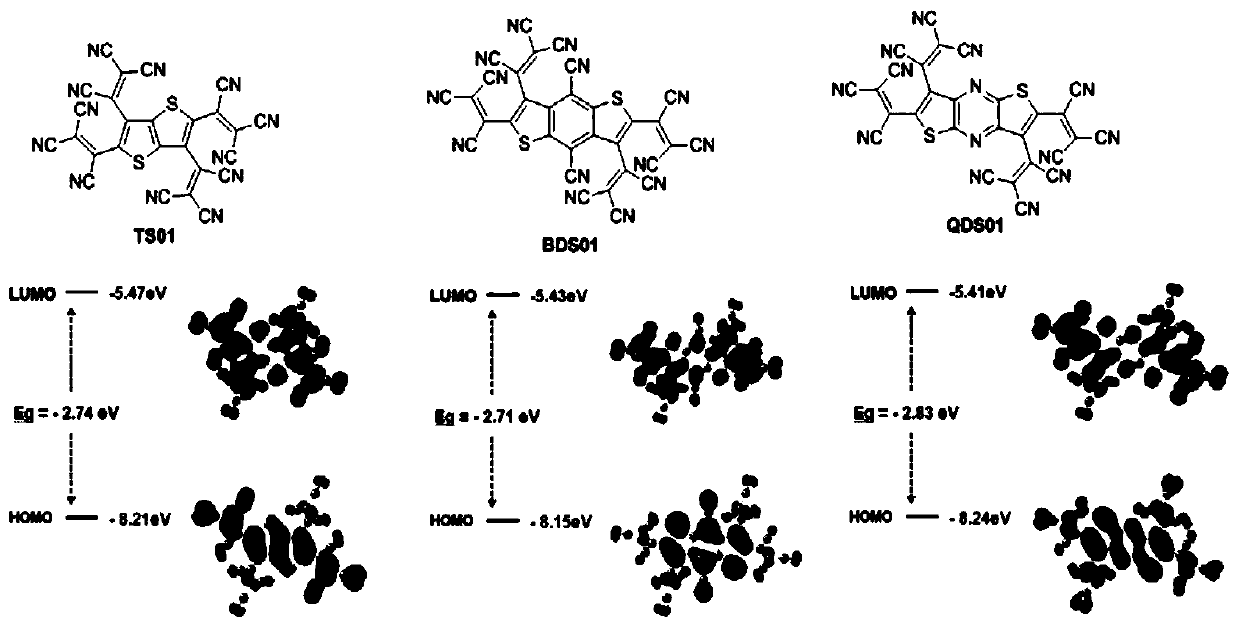
Dithiophene compound as well as preparation method and application thereof
A compound, dithiophene technology, applied in the field of dithiophene compound and its preparation and application, can solve the problems of poor electrical properties, difficult synthesis, poor stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] This embodiment provides a dithiophene compound intermediate compound, which has the structure shown in the following formula P01:
[0067]
[0068] The synthetic route of the intermediate compound of dithiophene compound shown in formula P01 is as follows:
[0069]
[0070] The preparation method of the dithiophene compound intermediate compound shown in formula P01 specifically comprises the following steps:
[0071] Take a 500ml double-neck round bottom bottle and put it into a stirring bar, fill it with nitrogen after drying; first add 14.022g of thieno
[0072] [3,2-b]thiophene (0.1mol, 1eq) and 36.488g N-bromosuccinimide (0.205mol, 2.05eq), followed by
[0073] Add 250mL of N,N-dimethylformamide and continue the reaction for 3 hours; stop the reaction with 200mL of saturated aqueous sodium bicarbonate solution, then extract with 200mL of diethyl ether, and repeat the extraction for 3 times, and the obtained extracts are sequentially added to magnesium sulfa...
Embodiment 2
[0077] This embodiment provides a dithiophene compound intermediate compound, which has the structure shown in the following formula P02:
[0078]
[0079] The synthetic route of the intermediate compound of the dithiophene compound shown in formula PO1 is as follows:
[0080]
[0081] The preparation method of the dithiophene compound intermediate compound shown in formula P02 specifically comprises the following steps:
[0082] (1) Synthetic intermediate C01
[0083] Take a 500 ml three-neck round bottom bottle and put it into a stirring bar and an upper addition funnel, fill it with nitrogen after drying; Dissolve 23.841g of compound P01 (0.08mol, 1eq) in 150mL of anhydrous tetrahydrofuran, and add the resulting solution dropwise into a three-neck round bottom flask at -78°C, and continue the reaction for 18 hours after the reaction gradually returns to temperature; Saturated aqueous sodium bicarbonate solution was used to stop the reaction, followed by extraction w...
Embodiment 3
[0089] This embodiment provides a dithiophene compound intermediate compound, which has the structure shown in the following formula P03:
[0090]
[0091] The synthetic route of the intermediate compound of dithiophene compound shown in formula P03 is as follows:
[0092]
[0093] The difference between the preparation method of the dithiophene compound intermediate compound shown in the formula P03 and the preparation step of the intermediate compound of the dithiophene compound shown in P01 provided in Example 1 is only:
[0094] Replace thieno[3,2-b]thiophene in Example 1 with 19.028g 4,8-dichloro-benzo[1,2-b:4,5-b']dithiophene (0.082mol, 1eq), And adjust other reactants to be identical with the molar equivalent in embodiment 1, obtain 37.942g compound P03 (productive rate: 91%)
[0095] Elemental analysis: (C 10 h 2 Br 2 Cl 2 S 2 ) Theoretical value: C, 28.81; H, 0.48; Br, 38.33; Cl, 17.00; S, 15.38; found value: C, 28.85;
[0096] HRMS (EI) m / z (M+): theor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com