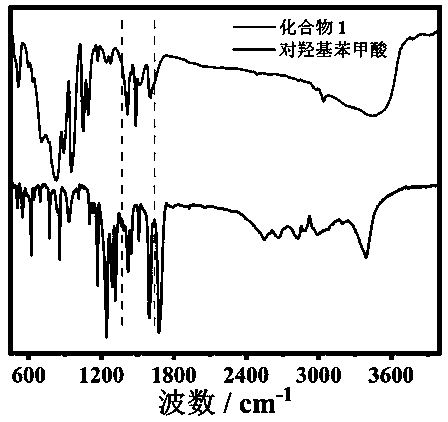
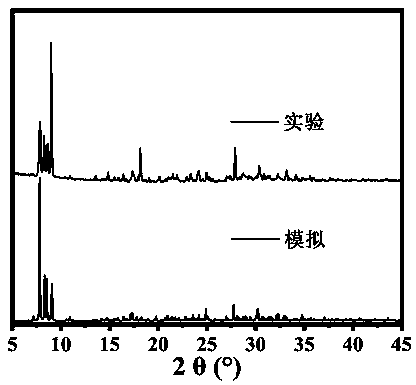
Organic-inorganic heteropolyacid-based rare earth derivative as well as preparation method and application thereof
A derivative and organic technology, applied in the field of preparation of polyacid-based fluorescent probe materials, can solve the problems of low water solubility and limit metal ions, and achieve the effect of preventing hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] An example of organic-inorganic hybrid multi-acid-based rare earth derivatives, the molecular formula of the multi-acid-based rare earth derivatives is [N(CH 3 ) 4 ] 3 K 2 [Eu(C 7 h 5 o 3 )(H 2 O) 2 (α-PW 11 o 39 )]·7H 2 O.
[0035] The preparation method of the above-mentioned organic-inorganic hybrid polyacid-based rare earth derivatives specifically comprises the following steps:
[0036] 1) EuCl 3 ∙6H 2 O (0.228 g, 0.600 mmol), ligand p-hydroxybenzoic acid (0.280 g, 0.200 mmol), polyacid precursor K 14 [P 2 W 19 o 69 (H 2 O)]∙24H 2 Add O (2.120 g, 0.465 mmol) into 30 mL of distilled water, stir until completely dissolved, adjust the pH value to 4.5±0.2 with 3 mol / L KOH aqueous solution, and stir at room temperature for 25 min;
[0037] 2) Put the solution obtained in step 1) into a water bath at 60°C, stir and heat for 2 hours, then add tetramethylammonium chloride (0.110 g, 1.000 mmol) while it is hot and stir for 25 minutes. After the reaction i...
Embodiment 2
[0045] Detect the solution concentration of compound 1 at the optimum fluorescence intensity:
[0046] 1) Compound 1 was formulated to a concentration of 1.0±0.1×10 -2 mol / L, 7.5±0.1×10 -3 mol / L, 5.0±0.1×10 -3 mol / L, 2.5±0.1×10 -3 mol / L, 1.0±0.1×10 -3 mol / L, 7.5±0.1×10 -4 mol / L, 5.0±0.1×10 -4 mol / L, 2.5±0.1×10 -4 mol / L, 1.0±0.1×10 -4 mol / L, 7.5±0.1×10 -5 mol / L, 5.0±0.1×10 -5 mol / L, 2.5±0.1×10 -5 mol / L, 1.0±0.1×10 -5 mol / L solution;
[0047] 2) The concentration of test compound 1 is 1.0±0.1×10 -3 Fluorescence emission spectrum and fluorescence excitation spectrum at mol / L, to determine the characteristic excitation peak and emission peak of compound 1, and optimize the detection conditions;
[0048] 3) Under the same test conditions, the test concentration is 1.0±0.1×10 -2 mol / L, 7.5±0.1×10 -3 mol / L, 5.0±0.1×10 -3 mol / L, 2.5±0.1×10 -3 mol / L, 1.0±0.1×10 -3 mol / L, 7.5±0.1×10 -4 mol / L, 5.0±0.1×10 -4 mol / L, 2.5±0.1×10 -4 mol / L, 1.0±0.1×10 -4 mol / L, 7.5±0.1×10...
Embodiment 3
[0052] Take 2.0 mL with a concentration of 7.5±0.1×10 -4 The mol / L compound 1 solution was placed in a quartz cuvette, and its time-resolved spectrum was tested under the excitation light of 330nm. The results are shown in Figure 9 .
[0053] Figure 9 The concentration of compound 1 solution is 7.5±0.1×10 -4 Time-resolved spectra under 330nm excitation light at mol / L. It can be seen from the figure that at 108 μs, a strong fluorescence emission peak at 440 nm is attributed to the fluorescence emission of the p-hydroxybenzoic acid ligand; at 112 μs, weak fluorescence emission peaks at 592 and 619 nm are attributed to Eu 3+ Characteristic fluorescence emission of ions; weak fluorescence emission peaks at 580, 650 and 700 nm at 115 μs are also attributed to Eu 3+ The characteristic fluorescence emission of the ion; the fluorescence emission of the p-hydroxybenzoic acid ligand gradually decreases with time, and the Eu 3+ The fluorescence emission of the ion's characteristic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com