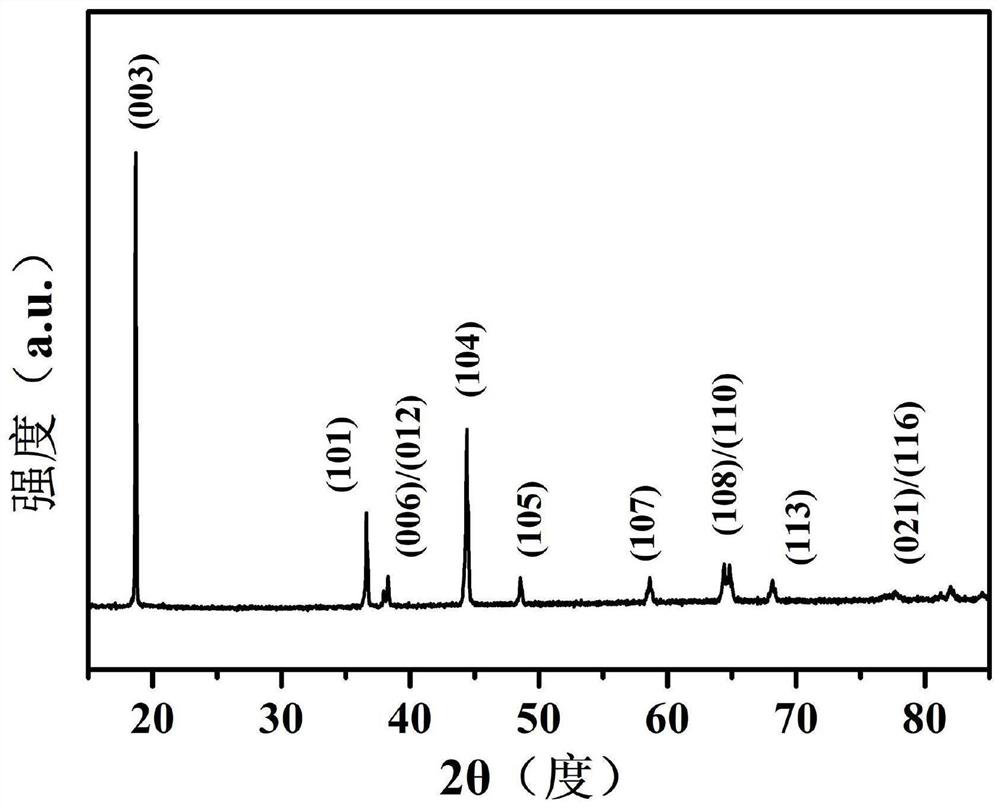
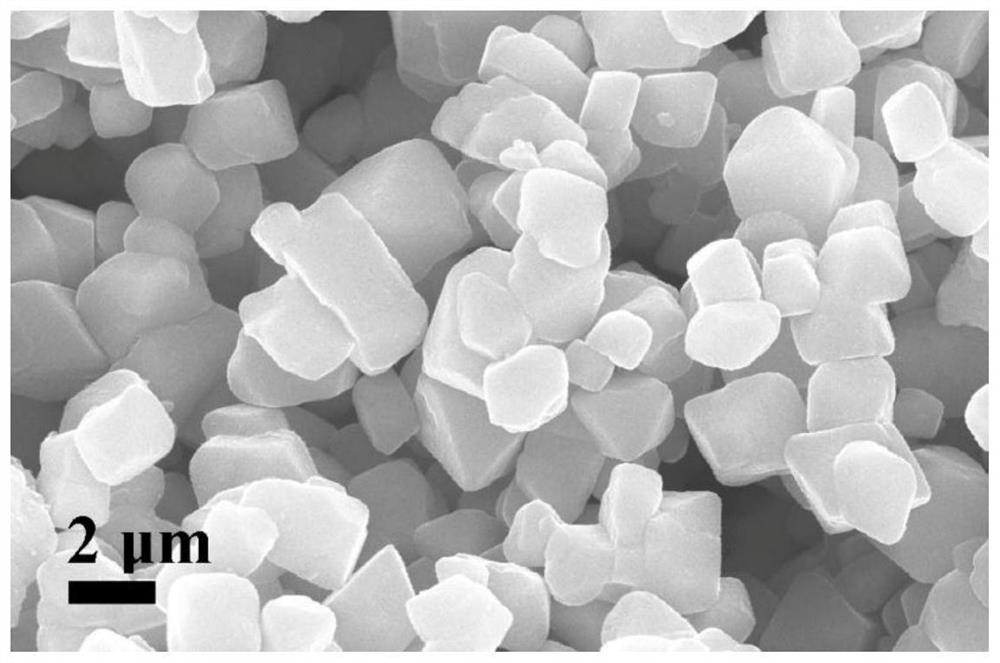
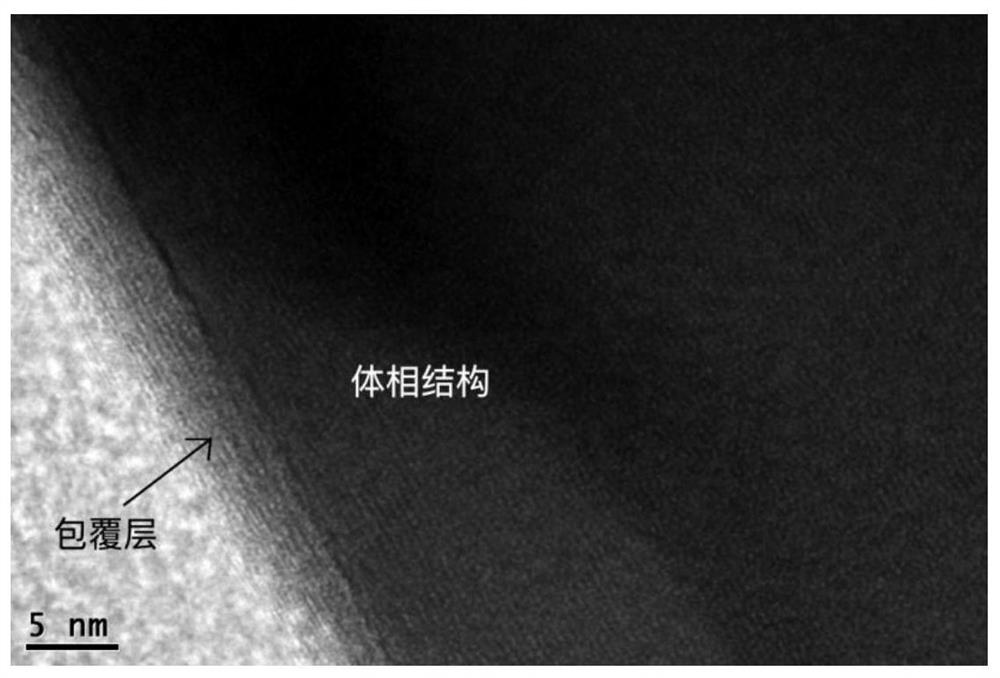
Method for preparing positive electrode material of core-shell structure lithium battery by secondary molten salt method
A technology of positive electrode material and core-shell structure, which is applied in the field of secondary molten salt preparation of core-shell structure lithium battery positive electrode material, can solve the problems of poor surface stability, high nickel ternary material capacity and voltage attenuation, etc., to reduce corrosion, Effect of improving long-life cycle performance and alleviating interfacial lattice mismatch
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 High nickel ternary single crystal LiNi 0.7 co 0.15 mn 0.15 o 2 preparation of
[0037] 1) Configure NaCl-KCl-Na according to the mass ratio of 30%: 30%: 40% 2 SO 4 For molten salt, fully grind and mix in a dry environment for later use. The high nickel ternary material LiNi 0.7 co 0.15 mn 0.15 o 2The required raw materials are weighed according to the stoichiometric ratio of lithium hydroxide, nickel carbonate, cobalt carbonate, manganese carbonate, ground and mixed to obtain reactant 1. Wherein, the mass ratio of lithium hydroxide is 5% in excess of its stoichiometric ratio. The mass ratio of the molten salt to the target positive electrode material (core-shell structure lithium battery positive electrode material) is 5:1.
[0038] 2) Fully mix the molten salt with reactant 1, grind, and react at high temperature: react at 600°C for 3h, then continue to react at 900°C for 10h. After the reaction, the cooling rate was 60°C / h, and the temperature w...
Embodiment 2
[0040] Example 2 High nickel ternary material LiNi 0.8 co 0.1 mn 0.1 o 2 preparation of
[0041] 1) Configure NaCl-KCl-Na according to the mass ratio of 60%: 20%: 20% 2 SO 4 For molten salt, fully grind and mix in a dry environment for later use. The high nickel ternary material LiNi 0.8 co 0.1 mn 0.1 o 2 The required raw materials lithium hydroxide, nickel carbonate, cobalt carbonate and manganese carbonate are weighed according to the stoichiometric ratio, ground and mixed for later use. Lithium hydroxide mass is in excess of 5% than its stoichiometric required amount. The mass ratio of molten salt to target cathode material is 1.1:1
[0042] 2) Fully mix the molten salt and the reactants, grind them, and react at high temperature. React at 600°C for 3h, then continue to react at 700°C for 10h. After the reaction, the cooling rate was 20°C / h, and the temperature was lowered to 500°C, followed by furnace cooling to obtain a reaction product, which was a mixture. ...
Embodiment 3
[0044] Example 3 High nickel ternary material LiNi 0.8 co 0.1 mn 0.1 o 2 preparation of
[0045] 1) Configure NaCl-KCl-Na according to the mass ratio of 40%: 20%: 40% 2 SO 4 For molten salt, fully grind and mix in a dry environment for later use. The high nickel ternary material LiNi 0.8 co 0.1 mn 0.1 o 2 The required raw materials lithium hydroxide, nickel carbonate, cobalt carbonate and manganese carbonate are weighed according to the stoichiometric ratio, ground and mixed for later use. Lithium hydroxide mass is in excess of 5% than its stoichiometric required amount. The mass ratio of the molten salt to the target cathode material is 2.0:1.
[0046] 2) Fully mix the molten salt and the reactants, grind them, and react at high temperature. React at 800°C for 3h, then continue to react at 750°C for 10h. After the reaction, the cooling rate was 50°C / h. After cooling down to 500°C, the reaction product was obtained by cooling in the furnace. The reaction product w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com