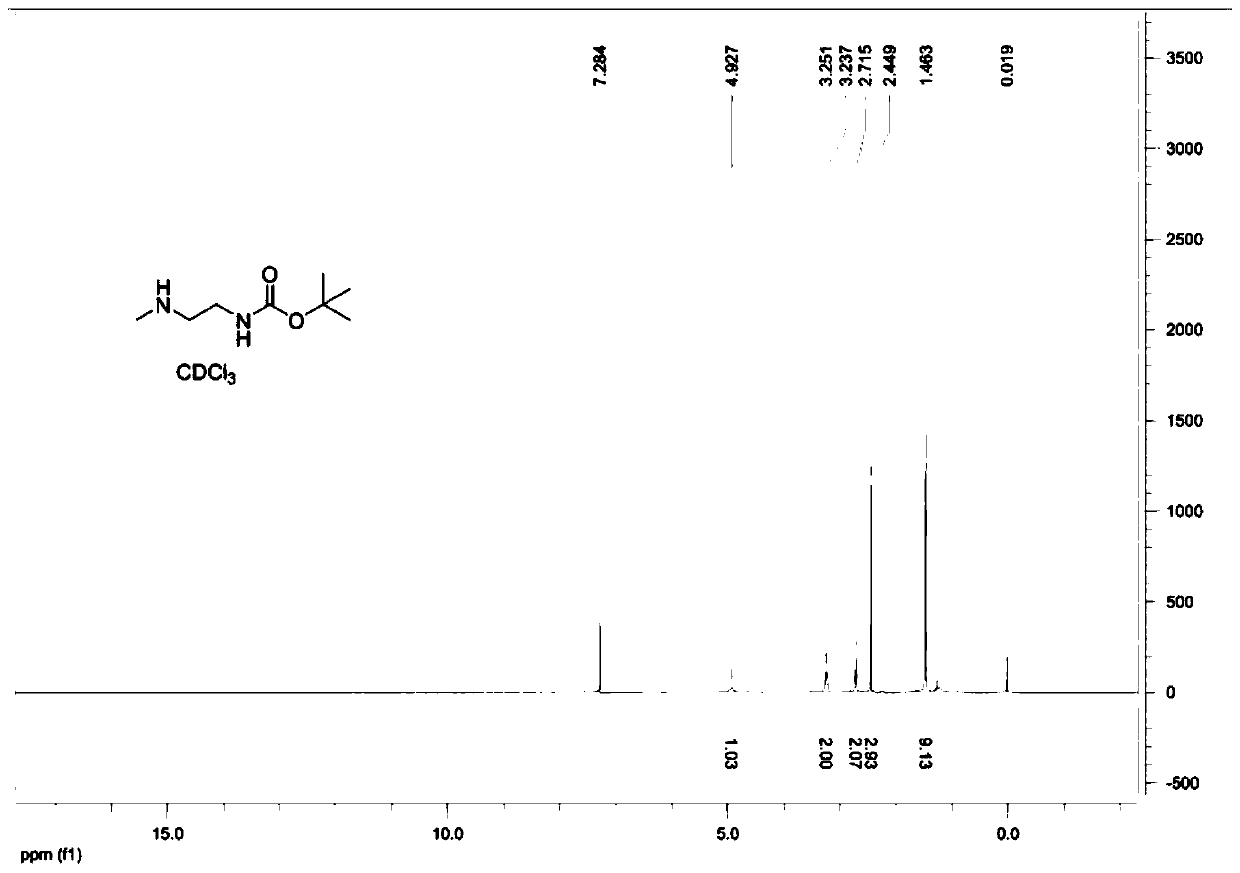
Preparation method of (2-methylamine-ethyl)-tert-butyl carbamate
A technology of carbamic acid and t-butyl carbamate, which is applied in the field of preparation of tert-butyl carbamate, can solve the problems of difficult operation, complex molecular structure, high cost, etc., and achieve the reduction of by-product content, fewer operation steps, and lower The effect of consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
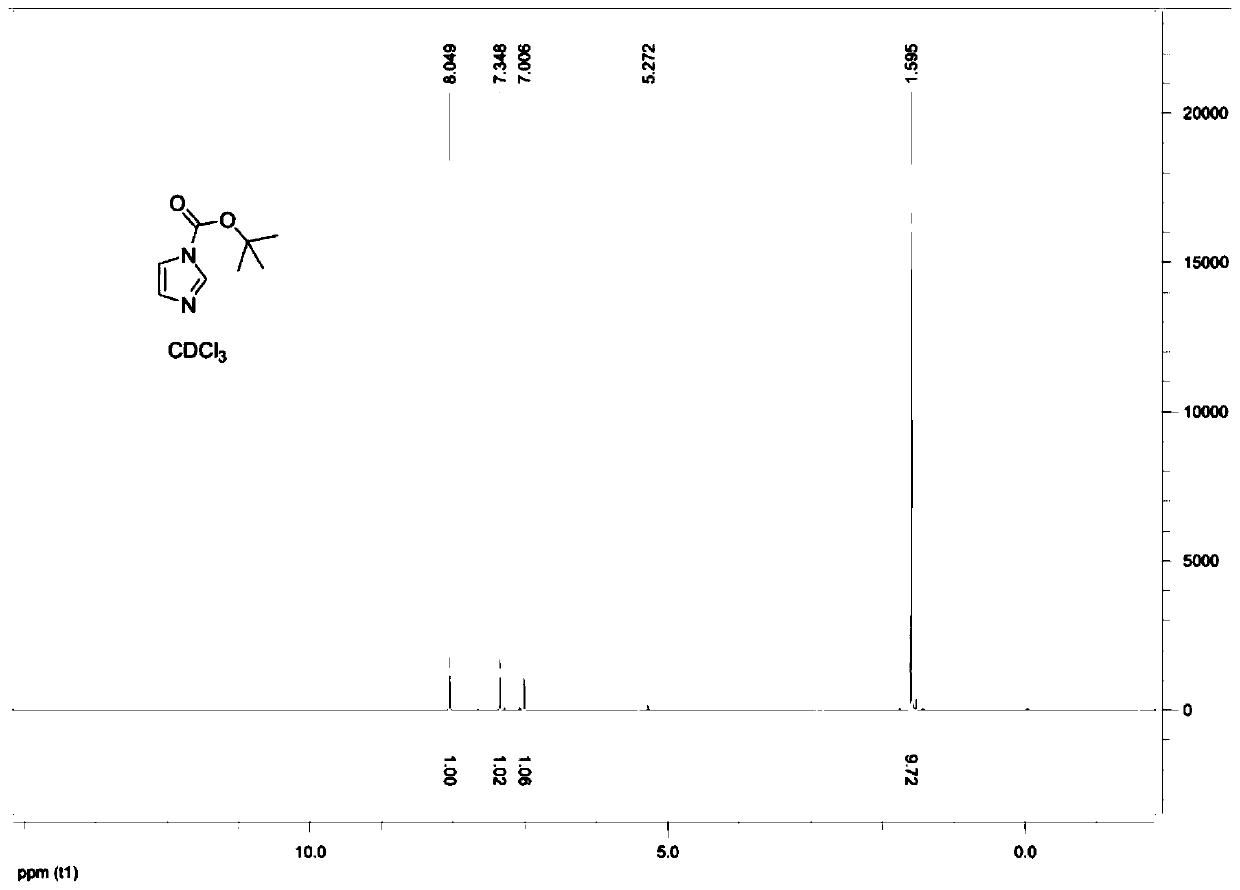
[0043] Embodiment 1: a kind of preparation method of (2-methylamine-ethyl)-tert-butyl carbamate comprises the following steps:
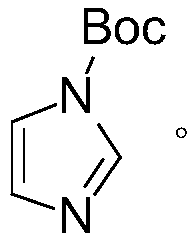
[0044] Step 1. Dissolve 75g of imidazole in 370ml of dichloromethane, and add 228.7g of di-tert-butyl dicarbonate at room temperature. After reacting at room temperature for 2 hours, wash with water three times, each dosage is 150ml; then use anhydrous Sodium sulfate is dried; Subsequent Buchner funnel filtration removes anhydrous sodium sulfate, and at 30 ℃, under the condition of vacuum tightness of 0.08MPa, adopting the mode of underpressure distillation to obtain the intermediate (solid) of 140g after removing methylene chloride ), the reaction equation is as follows:
[0045]
[0046]Step 2. Dissolve 51.6g of the intermediate in 90ml of toluene, then add 27.2g of N-methylethylenediamine, heat to 60°C, react for 2.5 hours, then distill off the toluene under reduced pressure; then add the 100ml dichloromethane as the organic phase, then add 10...
Embodiment 2
[0048] Embodiment 2: a kind of preparation method of (2-methylamine-ethyl)-tert-butyl carbamate, the difference with embodiment 1 is: the consumption of raw material, the temperature of reaction, vacuum degree and reaction time are different , its operation steps are as follows:
[0049] Step 1. Dissolve 75g of imidazole in 352.5ml of dichloromethane, and add 210g of di-tert-butyl dicarbonate at room temperature. After reacting at room temperature for 2 hours, wash with water three times, each dosage is 150ml, and then use anhydrous Sodium sulfate is dried, removes anhydrous sodium sulfate with Buchner funnel filtration subsequently, and at 35 ℃, under the condition of vacuum degree of 0.08MPa, adopt the mode of underpressure distillation to obtain the intermediate of 135g after removing dichloromethane ( solid).
[0050] Step 2. Dissolve 51.6g of the intermediate in 87.72ml of toluene, then add 25.8g of N-methylethylenediamine, heat to 70°C, react for 2 hours, then distill o...
Embodiment 3
[0051] Embodiment 3: a kind of preparation method of (2-methylamine-ethyl)-tert-butyl carbamate, the difference with embodiment 1 is: the consumption of raw material, the temperature of reaction, vacuum degree and reaction time are different , its operation steps are as follows:
[0052] Step 1. Dissolve 75g of imidazole in 390ml of dichloromethane, and add 240g of di-tert-butyl dicarbonate at room temperature, react at room temperature for 3 hours, wash with water three times, each dosage is 200ml, and then use anhydrous sulfuric acid Sodium is dried, then Buchner funnel filtration removes anhydrous sodium sulfate, and at 45 ℃, under the condition of vacuum degree of 0.1MPa, adopt the mode of underpressure distillation to obtain the intermediate (solid) of 142g after removing dichloromethane .
[0053] Step 2. Dissolve 51.6g of the intermediate in 103.2ml of toluene, then add 28.38g of N-methylethylenediamine, heat to 60°C, react for 4 hours, then distill off the toluene und...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com