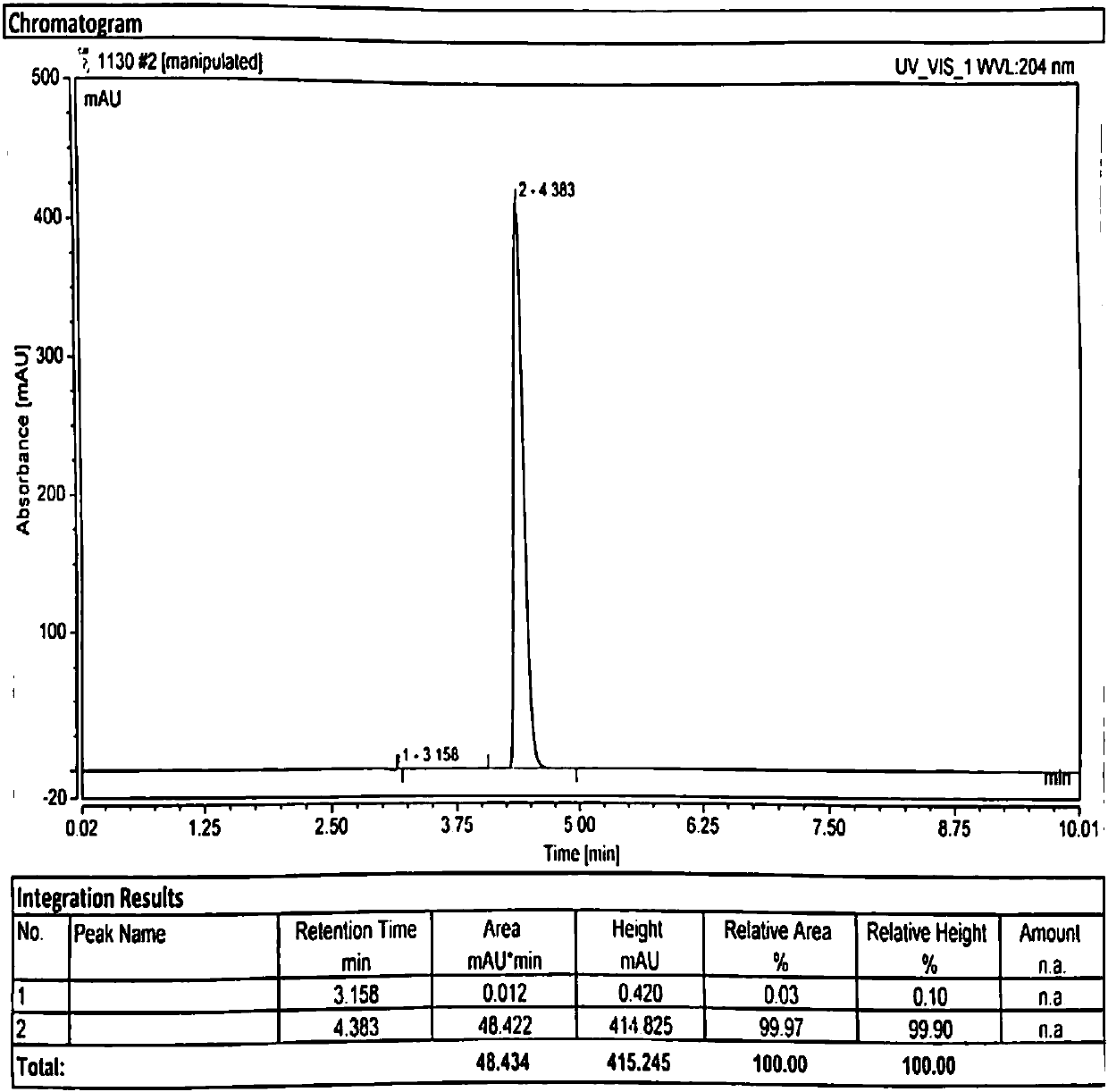
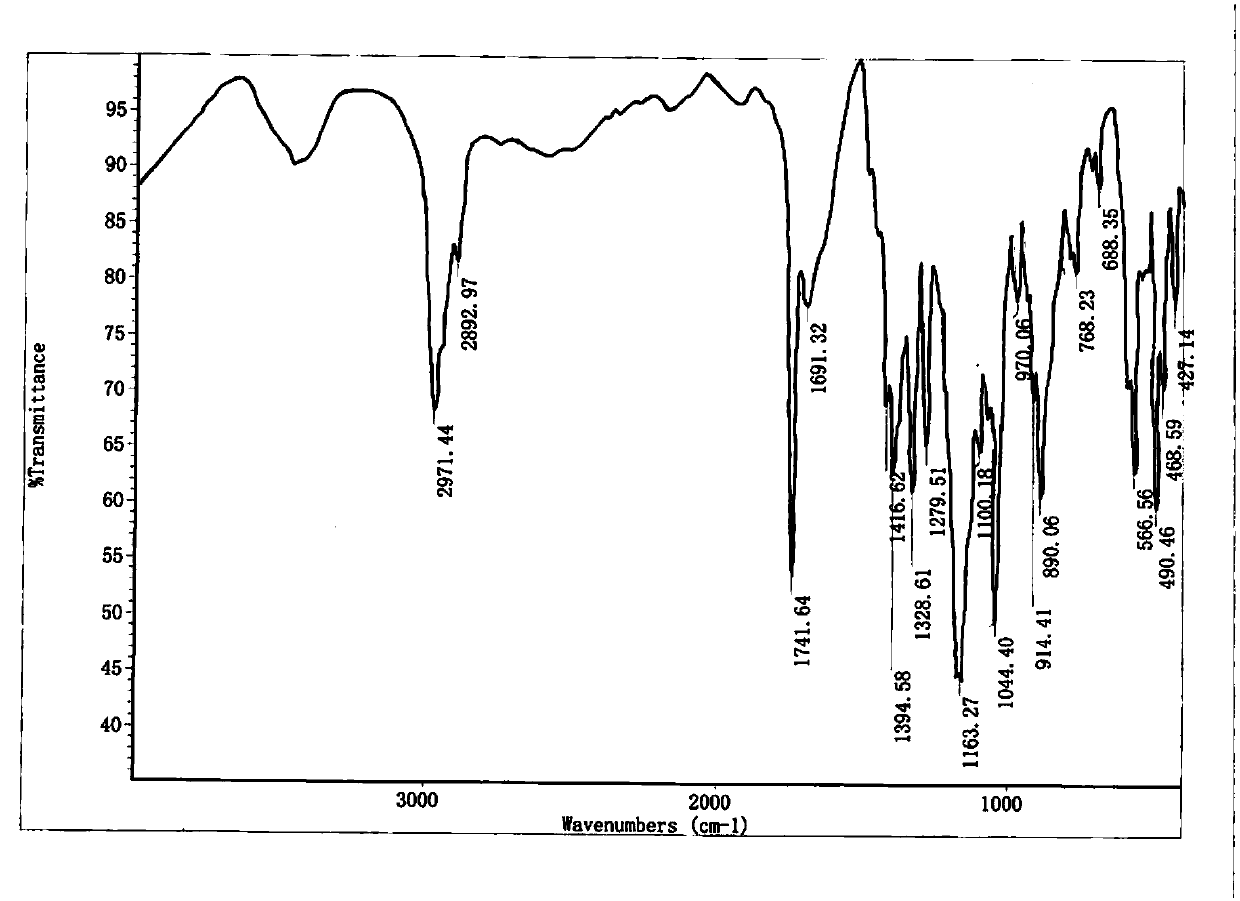
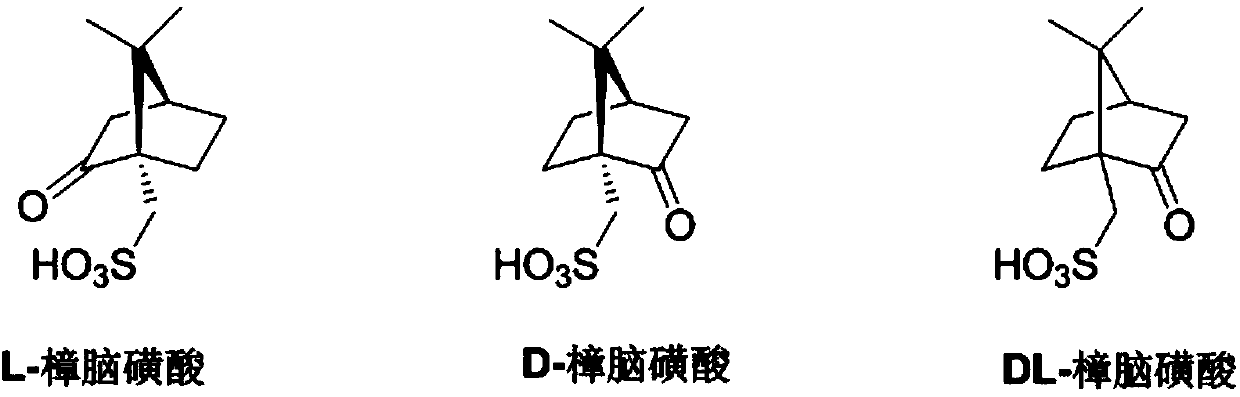
Method for recovering camphorsulfonic acid
A technology of camphorsulfonic acid and camphorsulfonate, which is applied in the field of medicine and chemical industry, can solve the problems of production process safety hazards, low recovery rate, air pollution, etc., and achieve the effects of low production cost, environmental friendliness and simple operation procedures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Add an aqueous solution containing camphorsulfonic acid to a 1L three-neck flask, and measure the pH. If the pH is not neutral, neutralize with concentrated hydrochloric acid or sodium carbonate. Add 0.01 (w / w) activated carbon, heat to 50-60°C and stir to react for 2 hours, then filter to remove activated carbon. The filtrate is concentrated under reduced pressure at 50-60°C to remove water, and the vacuum degree is ≤-0.08MPa. After the water is concentrated to dryness, lower the temperature to 20-30°C, add 3 times the concentrated solid weight of dichloromethane, beat the slurry and stir for 1 hour, then filter, and transfer the filter cake to an oven at 50-60°C to dry to constant weight. Transfer the dried solid to a 500ml three-neck flask, add 3 times the solid weight of absolute ethanol, dropwise add 1.1 times the equivalent of camphorsulfonate HCl / ethanol solution, control the temperature at 10-20°C, keep stirring for 1 hour, and pump After filtration, the filtr...
Embodiment 2
[0075] Add an aqueous solution containing camphorsulfonic acid to a 1L three-neck flask, and measure the pH. If the pH is not neutral, neutralize with concentrated hydrochloric acid or sodium carbonate. Add 0.02 (w / w) activated carbon, heat to 50-60°C and stir to react for 2 hours, then filter to remove activated carbon. The filtrate is concentrated under reduced pressure at 50-60°C to remove water, and the vacuum degree is ≤-0.08MPa. After the water is concentrated to dryness, lower the temperature to 20-30°C, add 3 times the concentrated solid weight of dichloromethane, beat the slurry and stir for 1 hour, then filter, and transfer the filter cake to an oven at 50-60°C to dry to constant weight. Transfer the dried solid to a 500ml three-neck flask, add 3 times the solid weight of absolute ethanol, dropwise add 1.1 times the equivalent of camphorsulfonate HCl / ethanol solution, control the temperature at 10-20°C, keep stirring for 1 hour, and pump After filtration, the filtr...
Embodiment 3
[0077] Add an aqueous solution containing camphorsulfonic acid to a 1L three-neck flask, and measure the pH. If the pH is not neutral, neutralize with concentrated hydrochloric acid or sodium carbonate. Add 0.10 (w / w) activated carbon, heat to 50-60°C and stir to react for 2 hours, and filter to remove activated carbon. The filtrate is concentrated under reduced pressure at 50-60°C to remove water, and the vacuum degree is ≤-0.08MPa. After the water is concentrated to dryness, lower the temperature to 20-30°C, add 3 times the concentrated solid weight of dichloromethane, beat the slurry and stir for 1 hour, then filter, and transfer the filter cake to an oven at 50-60°C to dry to constant weight. Transfer the dried solid to a 500ml three-neck flask, add 3 times the solid weight of absolute ethanol, dropwise add 1.1 times the equivalent of camphorsulfonate HCl / ethanol solution, control the temperature at 10-20°C, keep stirring for 1 hour, and pump After filtration, the filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com