Imidazole ionic liquid and synthesis method and application thereof
A technology of ionic liquid and synthesis method, applied in the preparation of carboxylate, organic chemistry, etc., to achieve the effect of less impurities and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Synthesis of [AVIM]Cl. Add 1 mol of N-vinylimidazole and 1.5 mol of allyl chloride, and simultaneously add 230 mL of acetonitrile into a three-necked round-bottomed flask, accompanied by mechanical stirring and circulating condensed water. The reaction was carried out at 70 °C for 24 h under the protection of nitrogen. Excess allyl chloride in the reaction was then removed by rotary evaporation, and unreacted N-vinylimidazole was extracted with ethyl acetate. Finally, it was dried in a vacuum oven for 48 hours to obtain viscous amber ionic liquid [AVIM]Cl.
[0028] 2. Synthesis of [AVIM][OAc]. With 1mol of 1-allyl-3-vinylimidazolium chloride and 2mol of potassium acetate, 340mL of isopropanol was added to the round bottom flask, stirred mechanically, and reacted at room temperature for 36h. Then remove excess potassium acetate and potassium chloride generated by the reaction by vacuum filtration, then remove excess isopropanol by rotary evaporation, then redissolv...
Embodiment 2
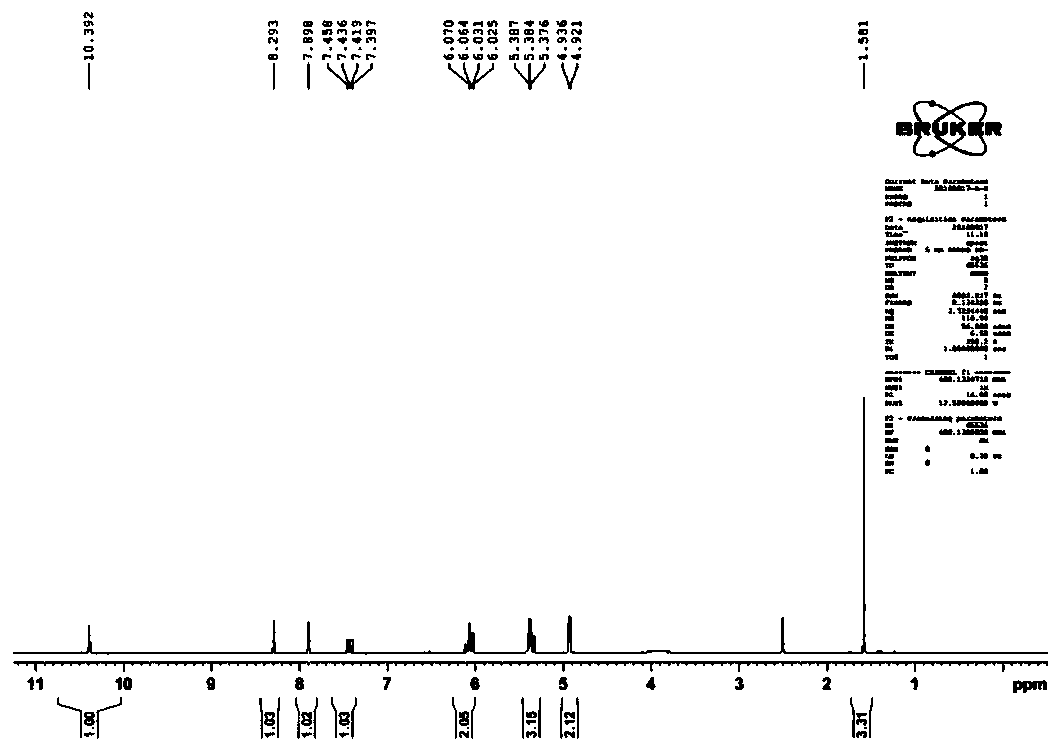
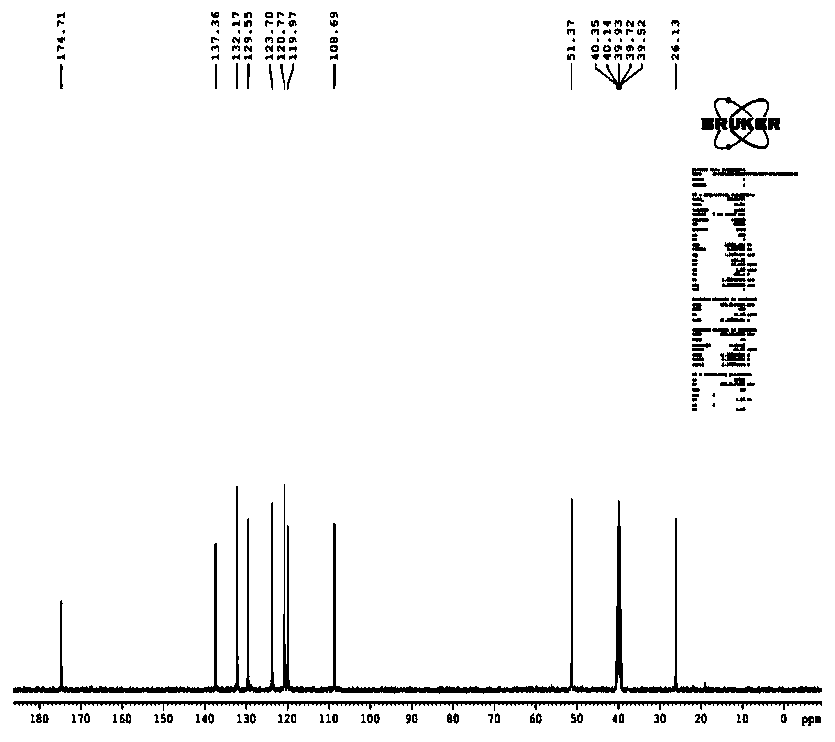
[0030] 1. The NMR characterization of [AVIM][OAc] prepared according to the method of Example 1 is as follows
[0031] 1 H NMR (400MHz, DMSO) δ10.38(s,1H),8.28(s,1H),7.88(s,1H),7.41(dd,J=15.7,8.8Hz,1H),6.19–5.96(m, 2H), 5.35(ddd, J=12.5, 9.9, 1.7Hz, 3H), 4.91(d, J=6.0Hz, 2H), 1.57(s, 3H)ppm.
[0032] 13 C NMR: (400MHz, D2O-d 2 )δ: 134.54, 130.09, 128.30, 123.11, 122.00, 119.63, 109.62, 51.98. [AVIM][OAc] NMR characterization spectrum, please refer to the appendix figure 1 ,2.
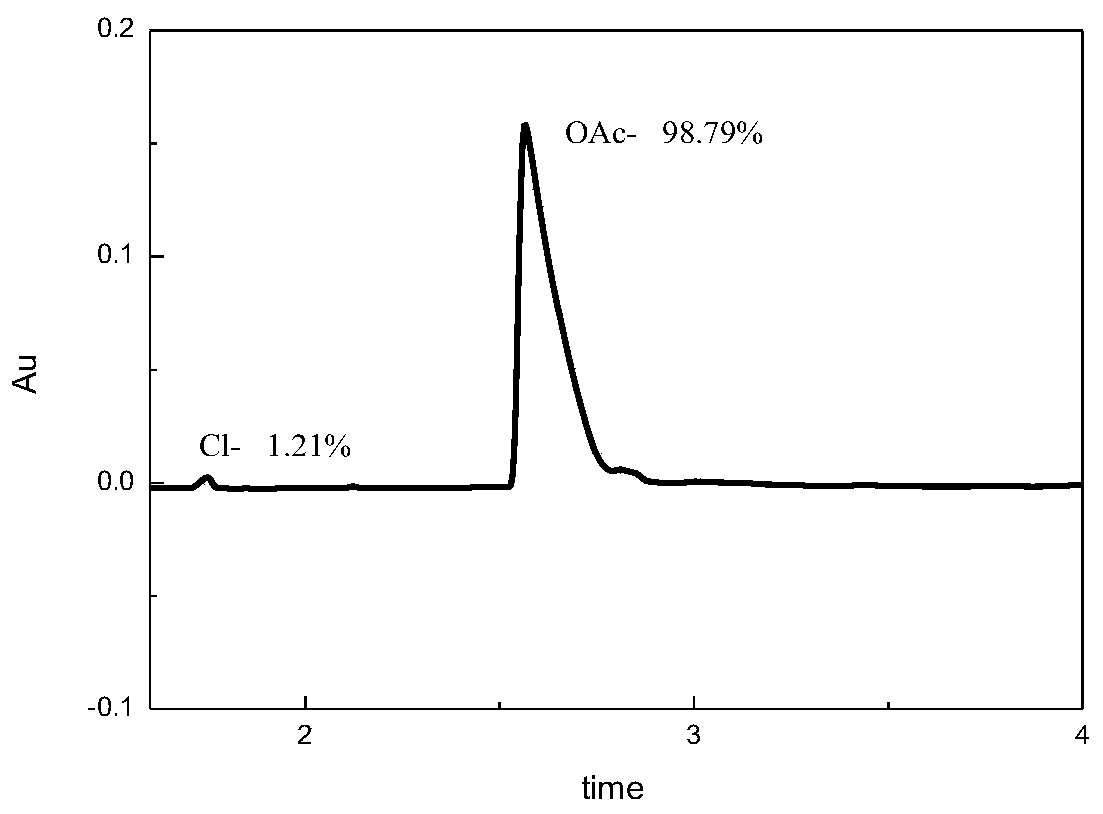
[0033] 2. Capillary electrophoresis analysis of [AVIM][OAc] prepared according to the method of Example 1 (content of acetate: 98.79%)
[0034] The acetate ion capillary electrophoresis curve of [AVIM][OAc] is shown in the appendix image 3
[0035] 3. The infrared spectrum analysis of [AVIM][OAc] prepared according to the method of Example 1
[0036] By infrared spectroscopy (attached Figure 4 ) can see the characteristic peaks of ionic liquids. 3086.9cm -1 Represents the stretching vibrat...
Embodiment 3
[0038] The new ionic liquid [AVIM][OAc] prepared according to the method of Example 1 is applied to the dissolution of biomacromolecules
[0039] 1. [AVIM][OAc] is applied to the dissolution of starch
[0040] Mix water and [AVIM][OAc] into molar ratios of 8 / 1, 6 / 1, 4 / 1, 2 / 1, 1 / 1 and pure ionic liquids. Mix the mixed solution of water and [AVIM][OAc] with cornstarch at a ratio of 3 / 1 (V / W), dissolve at 28°C for 1 hour, see attached Figure 5 . More than 90% of corn starch is dissolved in pure ionic liquid. From Figure 5 It can be seen that in the two pictures a and b, the biggest difference is that the molar ratio of water to [AVIM][OAc] is 4 / 1, and the starch can be completely dissolved at room temperature, while the fibers can be seen in other ratios Plain white precipitate. It shows that when the molar ratio of water and [AVIM][OAc] is 4 / 1, ionic liquid can dissolve starch well, which is better than other molar ratios.
[0041] 2. [AVIM][OAc] is applied to the dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com