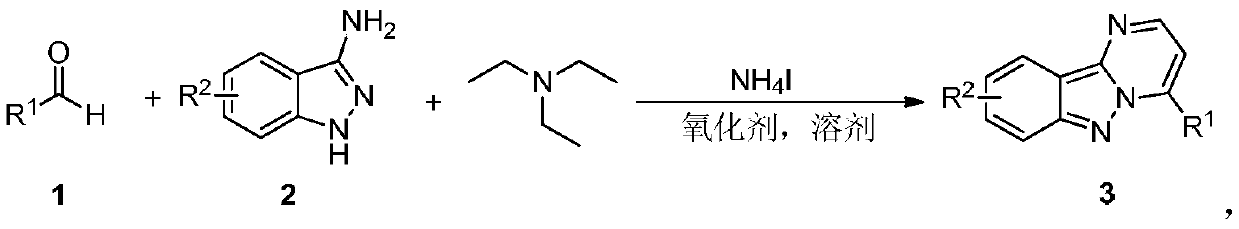
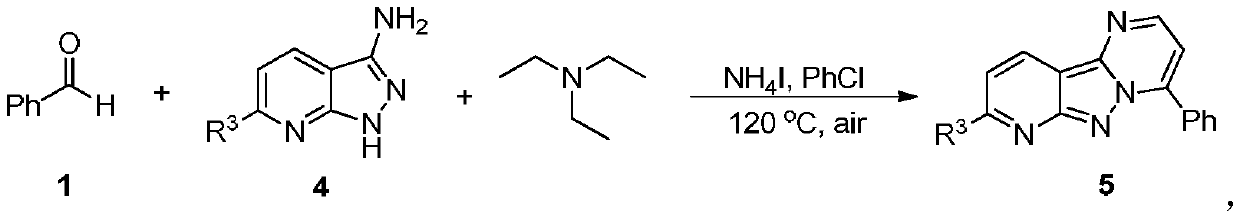
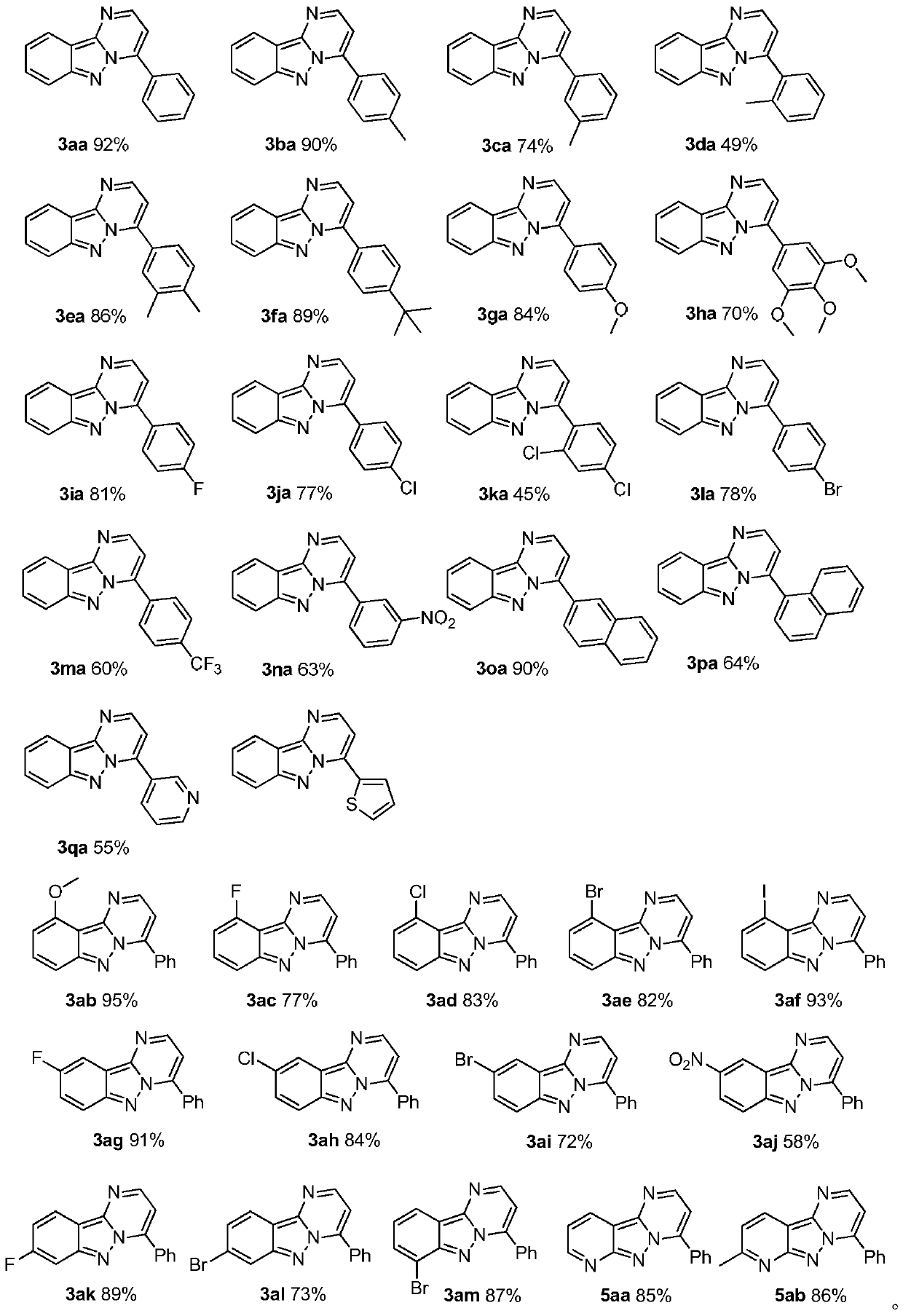
Synthesis method of pyrimido-indazole compound
A synthetic method and compound technology, applied in the direction of organic chemistry, can solve the problems of harsh reaction conditions, expensive catalysts, cumbersome post-treatment, etc., and achieve the effect of simple process, easy operation, avoiding waste of resources and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018]
[0019] Add benzaldehyde 1a (53mg, 0.5mmol), 3-aminoindazole 2a (66.5mg, 0.5mmol), triethylamine (126mg, 1.25mmol), ammonium iodide (108.8mg, 0.75mmol) into a 35mL reaction flask and chlorobenzene (2mL), then placed in an oil bath at 120°C and stirred for 12h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=15 / 1) to obtain the yellow solid product 3aa (112.7 mg, 92%). The characterization data of this compound are as follows: 1 H NMR (400MHz, CDCl 3 ): δ (ppm) 8.68 (d, J = 4.4Hz, 1H), 8.36 (d, J = 8.4Hz, 1H), 8.25–8.16 (m, 2H), 7.87 (d, J = 8.8Hz, 1H) ,7.67–7.57(m,4H),7.34(d,J=7.2Hz,1H),7.31–7.27(m,1H); 13 C NMR (100MHz, CDCl 3 )δ (ppm) 151.0, 145.2, 145.0 (4), 144.9 (8), 131.3...
Embodiment 2
[0021] Add 1a (53mg, 0.5mmol), 2a (66.5mg, 0.5mmol), triethylamine (126mg, 1.25mmol), ammonium iodide (72.5mg, 0.5mmol) and chlorobenzene (2mL) into a 35mL reaction flask, Then placed in an oil bath at 120°C and stirred for 12h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=15 / 1) to obtain the yellow solid product 3aa (100.4 mg, 82%).
Embodiment 3
[0023] Add 1a (53mg, 0.5mmol), 2a (66.5mg, 0.5mmol), triethylamine (126mg, 1.25mmol), ammonium iodide (72.5mg, 0.5mmol) and chlorobenzene (2mL) into a 35mL reaction flask, Then placed in an oil bath at 110°C and stirred for 12h. Add 50mL of water to quench the reaction, extract with ethyl acetate (50mL×3), and then the organic phase is treated with 10% Na 2 S 2 o 3 The solution was washed successively with saturated brine, and dried over anhydrous sodium sulfate. It was filtered, spin-dried, and separated by silica gel column (petroleum ether / ethyl acetate=15 / 1) to obtain the yellow solid product 3aa (75.9 mg, 62%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com