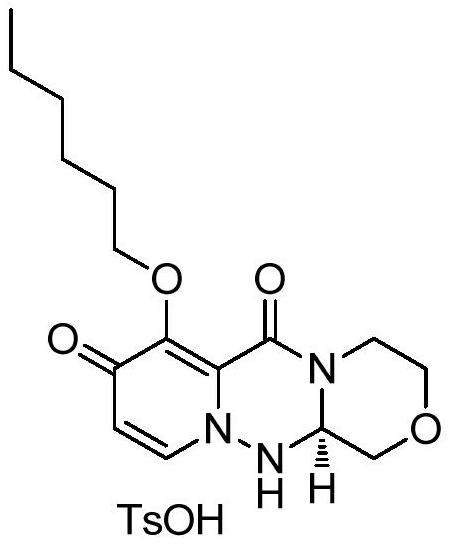
A kind of synthetic method of key mother core intermediate of baloxavir dipivoxil
A synthesis method and a technology for savidate are applied in the field of chemical synthesis of a new anti-influenza drug baloxavir dipivoxil core intermediate, which can solve the problems of low utilization rate of raw materials, low overall yield and high process cost, and achieves The effect of reducing the number of steps in the reaction route, high purity, and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
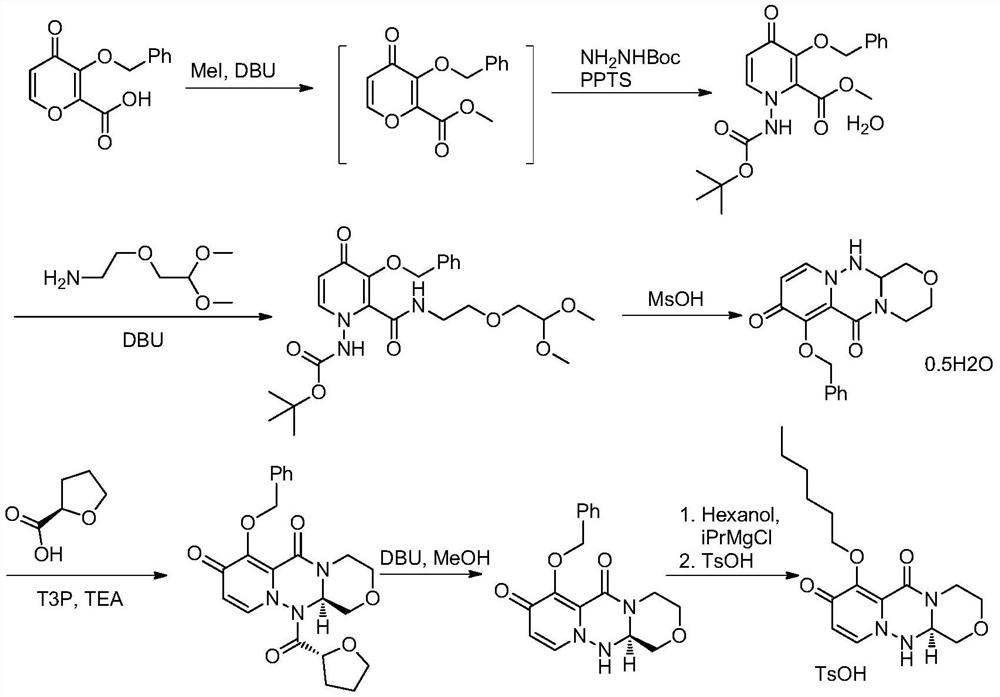
[0030] Add compound formula 1 (24.03g, 100mmol) and dichloromethane (120mL) into the three-necked flask, stir well, add triethylamine (20.24g, 200mmol), add EDCI (23.00g, 120mmol), and stir for 20 to 30 minutes A solution of 2-(2,2-dimethoxyethoxy)ethylamine compound formula 2 (16.41g, 110mmol) in dichloromethane (60mL) was added dropwise, and reacted at 25-30°C for 6-8 hours after the addition was completed. After the reaction, add water (240mL), separate the layers, extract the aqueous phase with dichloromethane (120mL) once, combine the organic phases, wash once with 2% dilute hydrochloric acid (120mL), wash once with saturated brine (120mL), anhydrous Dry over sodium sulfate, concentrate, beat with a mixed solvent of ethyl acetate and petroleum ether, filter, and dry to obtain product 3 (34.54g, 93.0%)
[0031] MS(ESI)m / z=372.2[M+H] + , 1 H NMR (400MHz, CDCl 3 )δ7.14(br,1H),7.82(d,J=6.0Hz,1H),6.48(d,J=5.6Hz,1H),4.40-4.55(m,1H),4.16-4.31(m,2H ), 3.45-3.60 (m...
Embodiment 2
[0034]
[0035] Add compound formula 3 (37.14g, 100mmol) and tetrahydrofuran (186mL) into the three-necked flask, add p-toluenesulfonic acid hydrate (3.80g, 20mmol), slowly add hydrazine hydrate (6.88g, 110mmol, 80%), after stirring Heat to 50-55°C and react for 4-6 hours. After the reaction was completed, slowly cool to room temperature, add 5% sodium bicarbonate solution (371mL) and stir, the aqueous phase was extracted twice with ethyl acetate (186mL), the combined organic phase was washed once with saturated brine (186mL), and dried over anhydrous sodium sulfate , filtered, concentrated and then slurried with a mixed solvent of ethyl acetate and petroleum ether, filtered and dried to obtain product 4 (32.25g, 91.0%).
[0036] The acid p-toluenesulfonic acid in embodiment 2 can be replaced by methanesulfonic acid, trifluoroacetic acid or trifluoromethanesulfonic acid; the reaction solvent tetrahydrofuran can be dichloromethane, toluene, acetonitrile, 2-methyltetrahydrofu...
Embodiment 3
[0038]
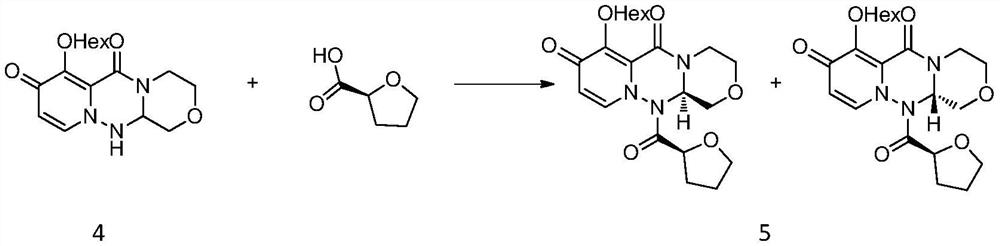
[0039]Add (S)-tetrahydrofuran-2-carboxylic acid (13.93g, 120mmol) and dichloromethane (160mL) into a three-necked flask, stir well and add, add pivaloyl chloride (15.68g, 130mmol), stir for 1 to 2 hours, then drop Add triethylamine (30.36g, 300mmol), and then add compound 4 (32.14g, 100mmol), and react at 25-30°C for 4-6 hours. Add water (160mL) after the reaction, separate the layers, extract the water phase with dichloromethane (80mL) twice, combine the organic phases with 10% sodium bicarbonate solution (160mL) and wash once, then wash twice with saturated brine (160mL) , dried over anhydrous sodium sulfate, concentrated and then recrystallized with a mixed solvent of acetone and petroleum ether, filtered and dried to obtain product 5 (18.41g, 43.9%).
[0040] MS(ESI)m / z=420.2[M+H] + , 1 H NMR (400MHz, CDCl 3 )δ7.12(d, J=7.6Hz, 1H), 6.38(d, J=8.0Hz, 1H), 5.90(dd, J=9.6, 2.4Hz, 1H), 4.65(d, J=13.6Hz, 1H),4.60(br,1H),4.16-4.31(m,2H),4.09-4.15(m,1H),3.90(t,J=6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com