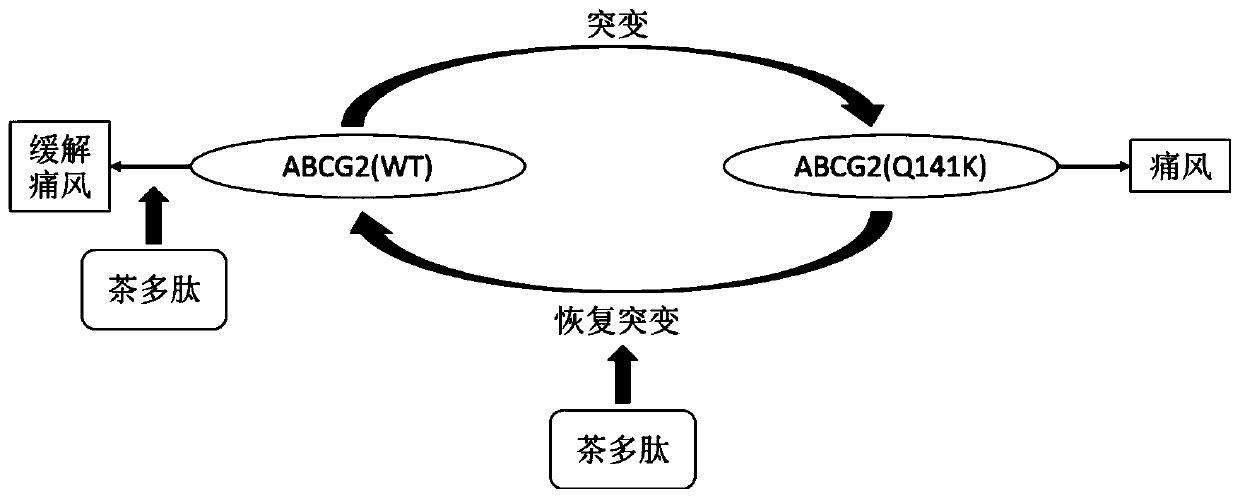
Application of tea polypeptides in the preparation of drugs to improve, alleviate or treat gout
A technology of polytea and medicine, which is applied in the pharmaceutical use of tea polypeptide, and the application field of tea polypeptide in the preparation, improvement, alleviation or treatment of gout drugs, which can solve the problems of easy drug resistance and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the preparation of tea polypeptide sample
[0059] 1. Preparation of tea protein:
[0060] S1. Wall breaking: use enzymatic hydrolysis technology to enzymatically hydrolyze and break the green tea tea dregs;
[0061] S2. Alkaline extraction: continue to extract green tea protein from the green tea dregs after enzymatic hydrolysis in step S1 by an alkaline method to obtain a green tea protein extract;
[0062] S3. Acid precipitation: acid precipitation is carried out on the green tea protein extract obtained in S2 to obtain a precipitate;
[0063] S4. Desalting: desalting the precipitate obtained in S3;
[0064] S5. Decolorization: take the desalted green tea protein in S4, use 75% acetone to decolorize, and freeze-dry to obtain green tea protein;
[0065] S6. Enzymolysis: the green tea protein obtained in S5 is added to food protease for enzymolysis to obtain a green tea protein polypeptide with blood pressure lowering activity.
[0066] Wherein, the g...
Embodiment 2
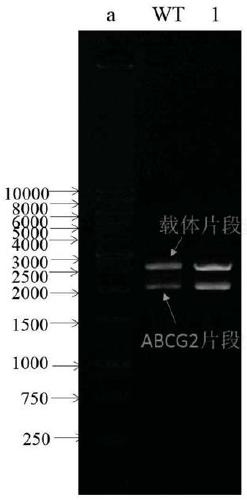
[0078] Example 2. Preparation and cell transfection of ABCG2 Q141K
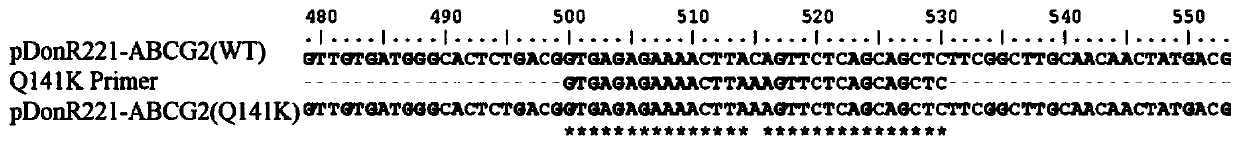
[0079] 1 site-directed mutagenesis
[0080] 1.1 Primer design
[0081] The design principles to be followed for site-directed mutagenesis are: change as few sites as possible, reduce mutation frequency, and be easy to verify. At the mutation site, a pair of reverse complementary primers was designed.
[0082] 1) Primer synthesis and desalting must be complete, and it is best to choose a purer purification scheme; 2) Primers generally need to be about 25-35bp, and the end of the primer ends with G or C; 3) Refer to the codon comparison table to minimize base changes; 4 ) There are at least 5-9 sites matching the template at both ends of the mutated site; 5) When designing primers, the restriction site on the mutation primer sequence can be considered, and the restriction site is preferably a vector and a gene fragment Restriction sites not found elsewhere. At the same time, the restriction site can be used...
Embodiment 3
[0119] Embodiment three, Western blot detection protein expression level
[0120] 1 Extraction of total cell protein
[0121] (1) Pretreatment of cells: by 37°C CO 2 Take out the 293T cells cultured in the cell culture plate in the incubator, discard the original culture medium, and wash the cell surface 3-4 times with 4°C pre-cooled 1×PBS to remove the serum and dead cells in the medium, and try as much as possible. Aspirate the remaining PBS in the cell culture dish. The washed cells were directly frozen and stored in a -80°C refrigerator for protein extraction. This method preserves cells for at least two weeks. (Note: If necessary, after washing the cells with PBS, it is not necessary to freeze them at -80°C, and you can directly proceed to step 2)
[0122] (2) Add PMSF-containing lysate: Take out the cell culture dish from the -80°C refrigerator, place it on ice, add PMSF-containing cell lysate to each of the above-mentioned culture dishes, and shake gently to make th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com