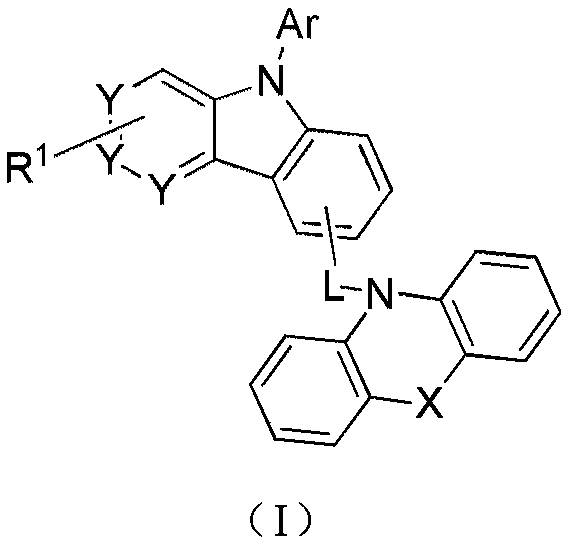
Compound and applications in organic electroluminescent field
A compound, unsubstituted technology, applied in the field of new organic compounds, can solve the problems of mismatch between electrons and holes in the light-emitting layer, efficiency roll-off, shortened lifespan, etc., achieve good hole and electron transport, prolong lifespan, and improve stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
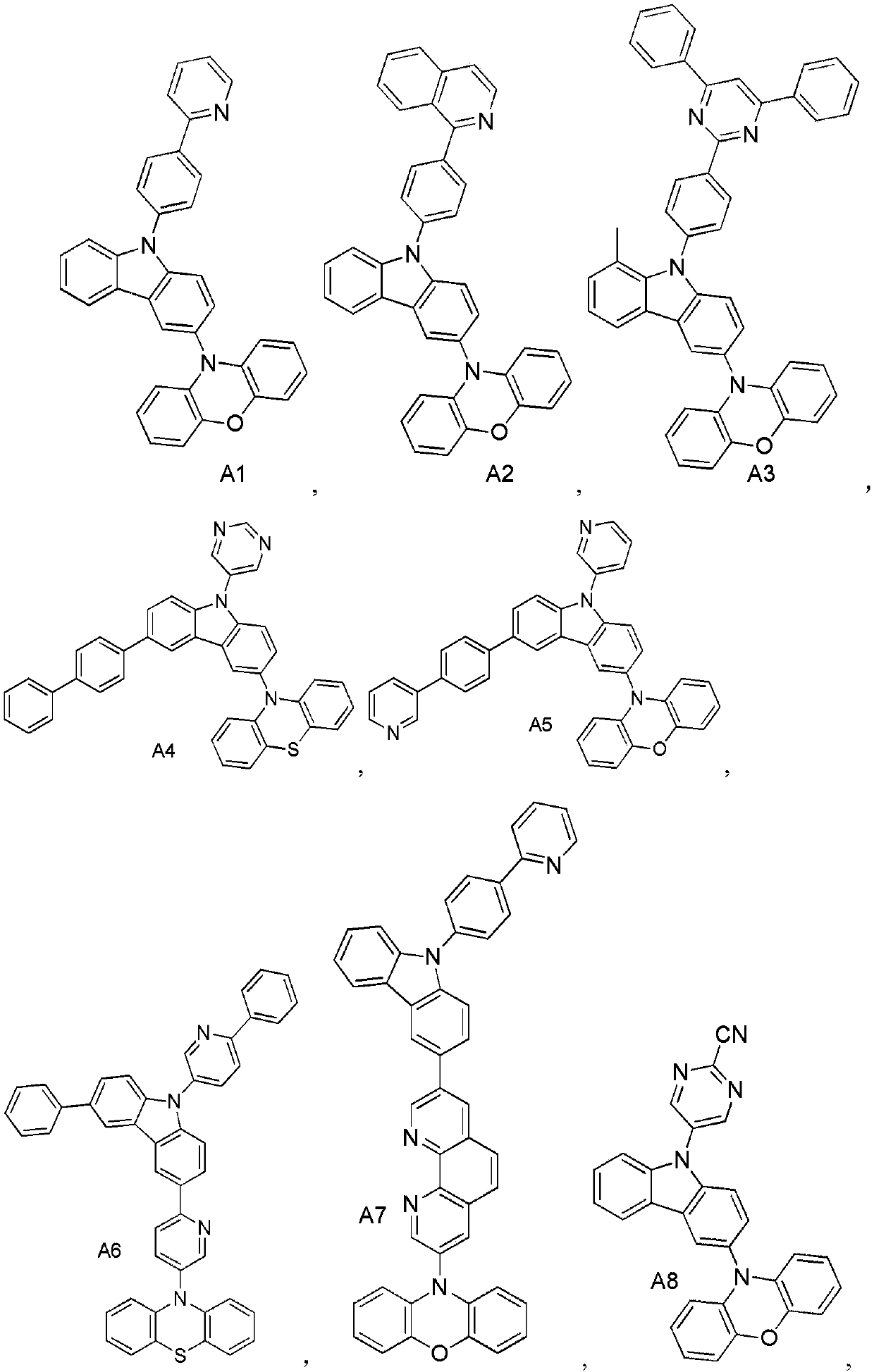
[0044] Synthesis Example 1: Synthesis of Compound A1
[0045]
[0046] Nitrogen protection, in the reaction flask, add 3.9g (17mmol) of 4-bromobenzene-2-(2-pyridyl), 3.6g (15mmol) of 3-bromocarbazole, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), toluene 1500ml, potassium carbonate 43.3g (31mmol), react at 100°C for 3.5h. The reaction was stopped, cooled to room temperature, filtered, and the resulting solid was purified by recrystallization in toluene to give intermediate M1.
[0047] Nitrogen protection, in the reaction flask, add intermediate M1 6.7g (17mmol), phenoxazine 3.5g (15mmol), Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), toluene 1500mL, potassium carbonate 43.3g (31mmol), react at 100°C for 3.5h. The reaction was stopped, cooled to room temperature, filtered, and the obtained solid was purified by recrystallization in toluene to obtain compound A1.
[0048] NMR data of compound A1:
[0049] 1H NMR (400MHz, Chloroform) δ8.59-8.02(m, 12H), 7.93(d, J=7.6Hz, 9H), 7.52(s, 3H),...
Synthetic example 2
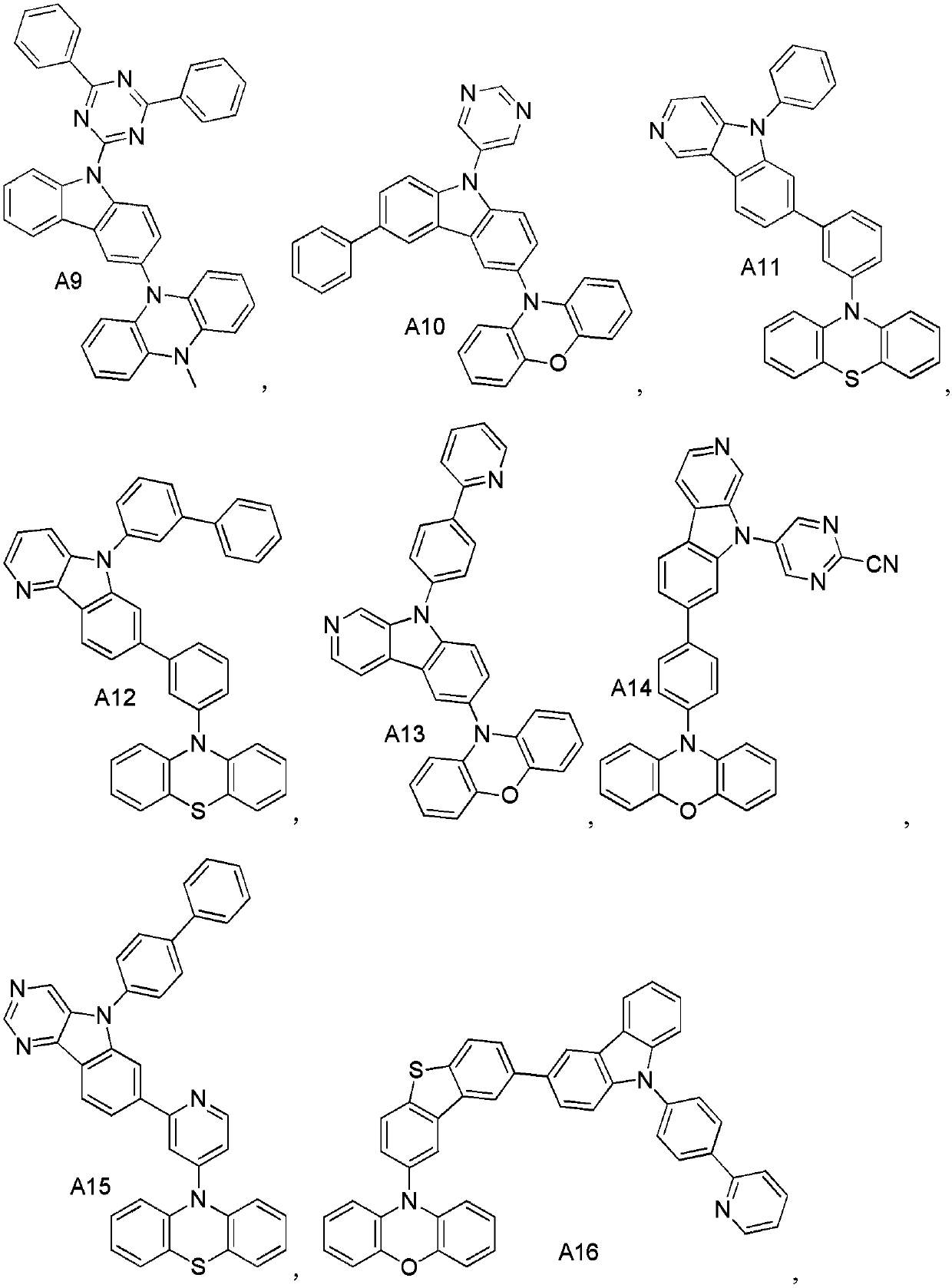
[0051] Synthesis Example 2: Synthesis of Compound A14
[0052]
[0053] Nitrogen protection, in the reaction flask, add 5.7g (17mmol) of 5-bromo-2-cyano-pyrimidine, 3.6g (15mmol) of 3-bromocarbazole, Pd 2 (dba) 3 0.9g (0.785mmol, 0.5%), toluene 1500mL, potassium carbonate 43.3g (31mmol), react at 100°C for 3.5h. The reaction was stopped, cooled to room temperature, filtered, and the resulting solid was purified by recrystallization in toluene to give intermediate M14-1.
[0054] In the reaction flask, add M2-128g (173mmol), p-chlorophenylboronic acid 30g (157mmol), tetrakis (triphenylphosphine palladium) 0.9g (0.785mmol, 0.5%), toluene 1500mL, ethanol 1000mL, potassium carbonate 43.3g ( 314mmol) / water 1000mL, react at 80°C for 3.5h. The reaction was stopped, cooled to room temperature, filtered and the resulting solid was purified by recrystallization from toluene to afford intermediate M14.
[0055] Nitrogen protection, in the reaction flask, add intermediate 7.6g M14 ...
Embodiment 1
[0078] The glass plate coated with the ITO transparent conductive layer is ultrasonically treated in a commercial cleaning agent, rinsed in deionized water, ultrasonically degreased in acetone: ethanol mixed solvent, baked in a clean environment until the water is completely removed, and then cleaned with ultraviolet light. Light and ozone cleaning, and bombardment of the surface with a beam of low-energy cations;
[0079] Place the above-mentioned glass substrate with an anode in a vacuum chamber, evacuate to 1×10-5~9×10-3Pa, and vacuum-deposit HT-11 on the above-mentioned anode layer film as a hole injection layer. The rate is 0.1nm / s, and the evaporation film thickness is 10nm;
[0080] On the hole injection layer, HT-5 was vacuum evaporated as the hole transport layer of the device, the evaporation rate was 0.1nm / s, and the total film thickness was 80nm;
[0081] The light-emitting layer of the device is vacuum-evaporated on the hole transport layer. The light-emitting la...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com