CoMn2O4 bimetallic oxide catalyst and preparation method and application
A bimetallic oxide and catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc. The problem of catalyst falling off or dissolution can achieve the effect of low synthesis cost, good stability and improved selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
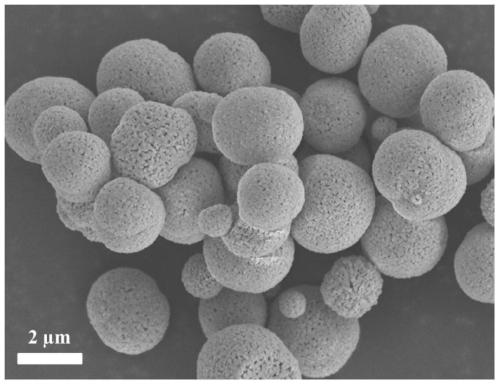
[0030] Weigh 0.73 g of cobalt nitrate hexahydrate and 1.26 g of manganese nitrate tetrahydrate and dissolve them in 40 mL of ethylene glycol and stir evenly. Add 20 mmol of urea as a precipitant to the mixture, stir it with magnetic force to make it fully mixed, and transfer the solution to In a hydrothermal reaction kettle, hydrothermal crystallization was carried out at 180 °C for 12 h. After the reaction was completed, it was cooled to room temperature. Calcined at 600 °C for 3 h in a Furnace to obtain CoMn 2 o 4 Bimetallic oxide catalysts.
[0031] 0.1 g of the catalyst was used for the selective oxidation of styrene. The reaction used tert-butyl hydroperoxide as the oxidant and acetonitrile as the solvent, and reacted at 80 °C for 6 h. The product was analyzed by gas chromatography. It was found that the conversion rate of styrene reached 97.7%, and the selectivity of styrene oxide reached 83.7%. %.
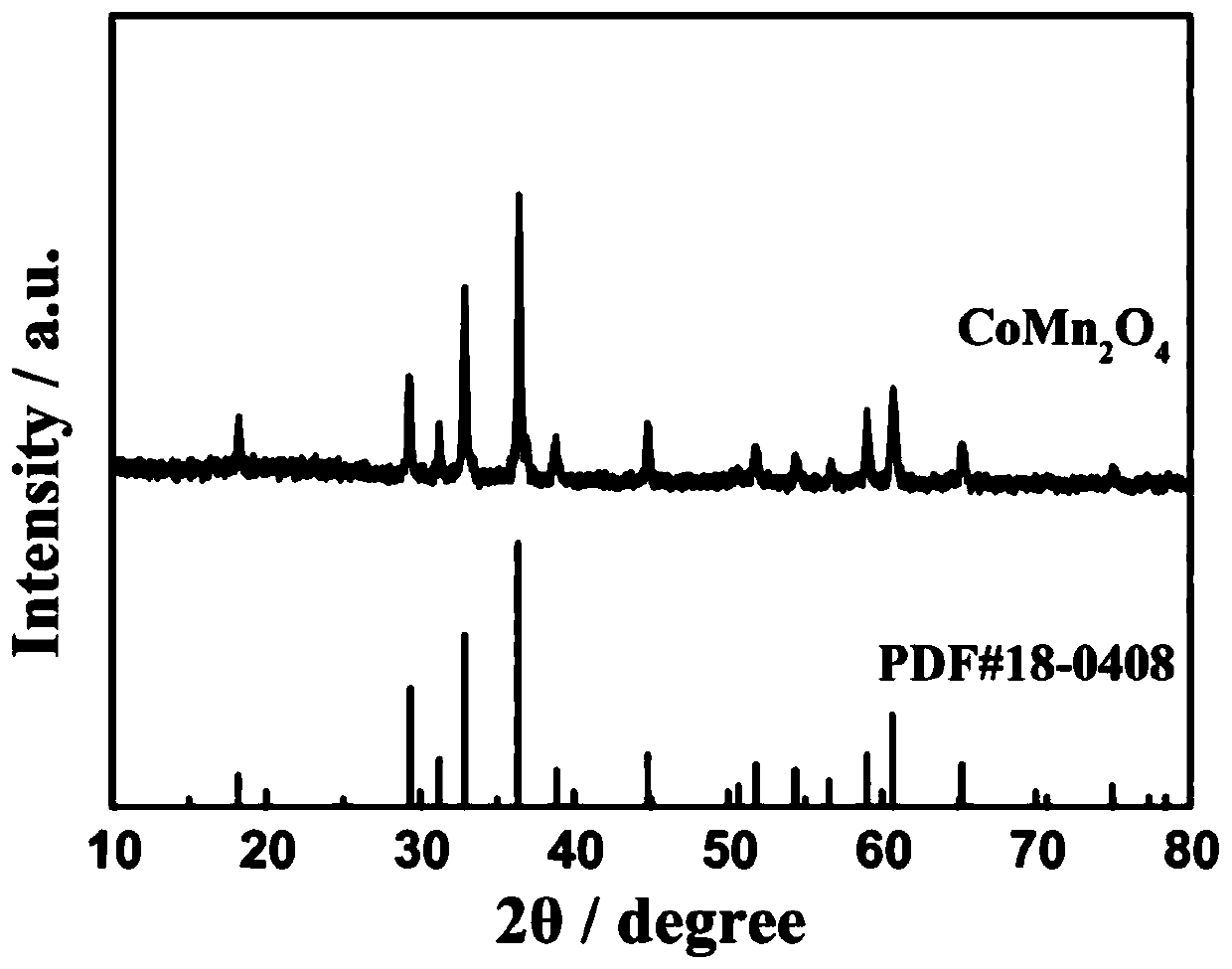
[0032] figure 1 For the prepared CoMn 2 o 4 X-ray diffraction p...
Embodiment 2
[0035] Repeat Example 1, except that the amount of urea used to prepare the catalyst is 25 mmol. The obtained catalyst was used in the oxidation reaction of styrene, and it was found that the conversion rate of styrene reached 90.9%, and the selectivity of styrene oxide reached 76.4%.
Embodiment 3
[0037] Example 1 was repeated, except that the calcination temperature used to prepare the catalyst was 500°C. The obtained catalyst was used in the oxidation reaction of styrene, and it was found that the conversion rate of styrene reached 99.2%, and the selectivity of styrene oxide reached 56.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com