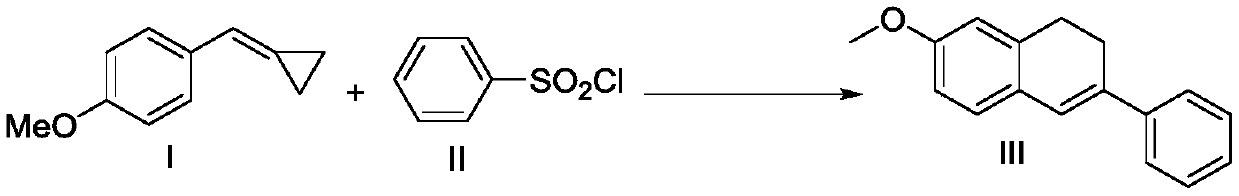Preparation method of 7-methoxy-3-phenyl-1,2-dihydronaphthalene
A technology of methoxyphenylmethylenecyclopropane and methoxy, applied in the field of preparation of 7-methoxy-3-phenyl-1,2-dihydronaphthalene, can solve the problem of low yield of target product , long synthetic route, high synthetic cost and other problems, to achieve the effect of low cost, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] To a Slenk lock reactor, add 4-methoxyphenylmethylenecyclopropane (48mg, 0.3mmol), benzenesulfonyl chloride (3eq, 158mg), Ru(bpy) 3 Cl 2 ·6H 2 O (11.2mg, 5mol%), Na 2 CO 3 (2eq, 63.6mg) and acetonitrile (2mL), then the reactor was replaced with nitrogen for 3 times, and reacted for 12 hours at room temperature and 24W energy-saving lamp lighting conditions, and the reaction was completely detected by TLC or GC, and then the reaction solution was filtered through filter paper The catalyst mixture was recovered and dried, the filtrate was concentrated in vacuo, and the residue was separated by silica gel column chromatography (the elution solvent was n-hexane / ethyl acetate mixed solvent, the volume ratio was 50:1) to obtain 7-methoxy-3- 64 mg of phenyl-1,2-dihydronaphthalene, yield 90.4%.
Embodiment 2
[0025] To a Slenk lock reactor, add 4-methoxyphenylmethylenecyclopropane (48 mg, 0.3 mmol), benzenesulfonyl chloride (3 eq, 158 mg), Eosin Y (9.7 mg, 5 mol%), Na 2 CO 3 (2eq, 63.6mg) and acetonitrile (2mL), then the reactor was replaced with nitrogen for 3 times, and reacted for 24 hours at room temperature and under 24W energy-saving lighting conditions, and the reaction was detected by TLC or GC. Column filtration, vacuum concentration, and the residue was separated by silica gel column chromatography (eluting solvent was n-hexane / ethyl acetate mixed solvent, volume ratio was 50:1) to obtain 7-methoxy-3-phenyl-1 ,2-Dihydronaphthalene 51.7mg, yield 73%.
Embodiment 3
[0027] To a Slenk lock reactor, add 4-methoxyphenylmethylenecyclopropane (48mg, 0.3mmol), benzenesulfonyl chloride (3eq, 158mg), Ir(ppy)3 (9.8mg, 5mol%) , Na 2 CO 3 (2eq, 63.6mg) and acetonitrile (2mL), then the reactor was replaced with nitrogen for 3 times, and reacted at room temperature and 5W LED blue light for 12 hours, and the reaction was complete by TLC or GC detection, and then the reaction solution was passed through silica gel for a short time Column filtration, vacuum concentration, and the residue was separated by silica gel column chromatography (eluting solvent was n-hexane / ethyl acetate mixed solvent, volume ratio was 50:1) to obtain 7-methoxy-3-phenyl-1 ,2-Dihydronaphthalene 57.3mg, yield 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com