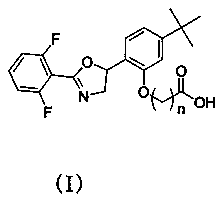
Etoxazole hapten, and synthesis method and application thereof
A synthesis method and etoxazole technology are applied in the field of etoxazole hapten and synthesis thereof, and achieve the effects of easy availability of raw materials, easy control of reaction conditions, and high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
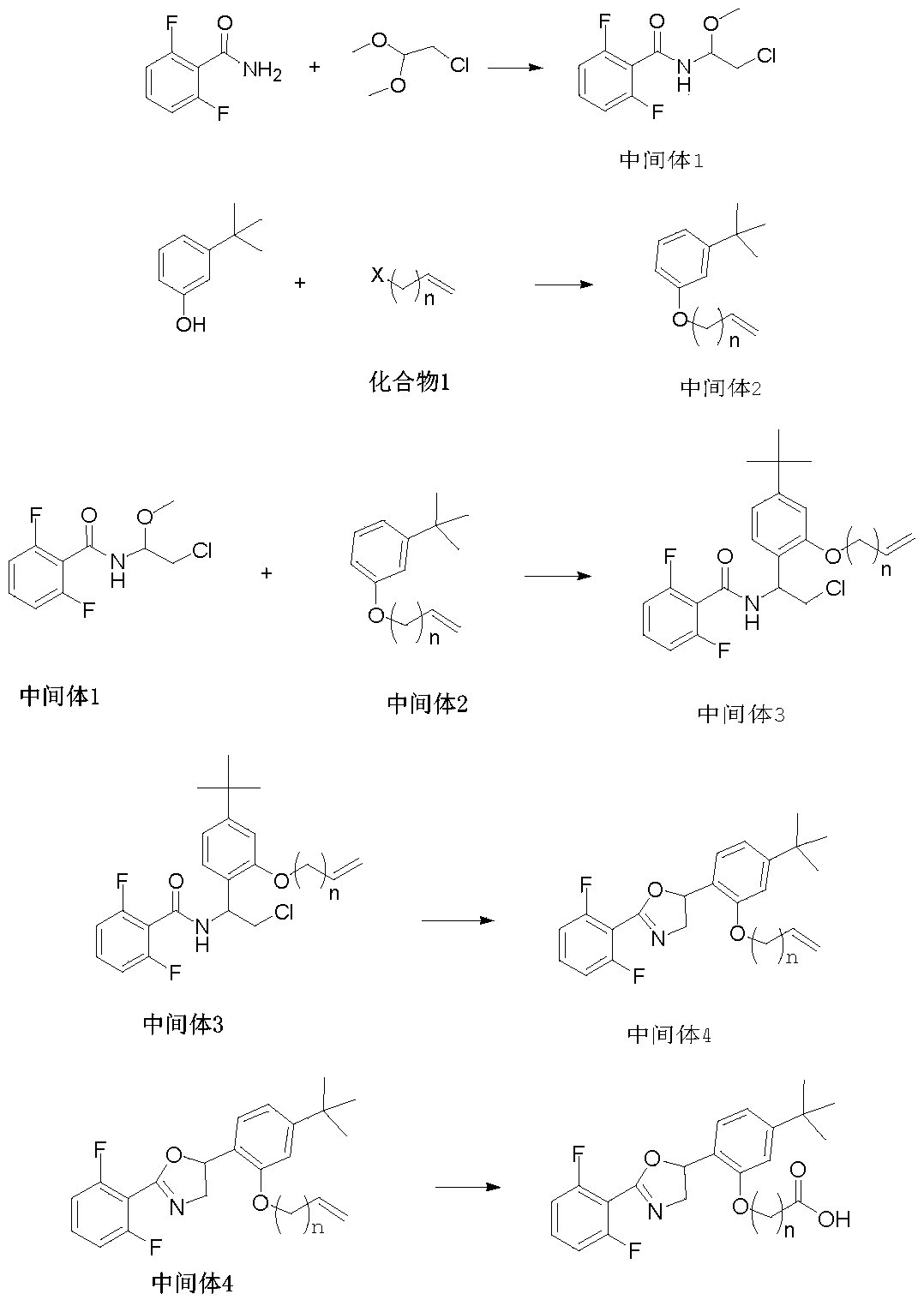
[0036] In the second aspect, the present invention also provides a method for synthesizing the above-mentioned etoxazole hapten, comprising the following steps:
[0037] S1, providing intermediate 1 having the structural formula ;
[0038] S2, providing an intermediate 2 having the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0039] S3, reacting the intermediate 1 and the intermediate 2 to obtain the intermediate 3, the intermediate 3 has the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0040] S4, subjecting the intermediate 3 to a condensation reaction to obtain the intermediate 4, the intermediate 4 has the structural formula , wherein, n is the number of -CH2 groups, and n is an integer of 1-5;
[0041]S5, performing an oxidation reaction on the intermediate 4 to obtain the etoxazole hapten.
[0042] According to the structural characteristics of etoxazole, the invention ...
Embodiment 1
[0058] A synthetic method of etoxazole hapten, comprising the steps of:
[0059] S1, providing intermediate 1, wherein the preparation method of intermediate 1 is: add 2,6-difluorobenzamide and 2-chloroacetaldehyde dimethyl acetal to a one-necked bottle, and stir for 5 minutes under ice-water bath conditions , then slowly add concentrated sulfuric acid to it, after the dropwise addition, react overnight at room temperature, after the reaction, pour the reaction solution into ice water, stir well, extract 2-3 times with ethyl acetate, combine the organic phases, organic After washing and concentrating, the phase is purified by column to obtain intermediate 1; wherein, the ratio of the amount of substances of the 2,6-difluorobenzamide, 2-chloroacetaldehyde dimethyl acetal and concentrated sulfuric acid is 1:1.5: 15;
[0060] S2, providing intermediate 2, wherein, the preparation method of intermediate 2 is: add 0.03mol 3-tert-butylphenol, 0.09mol potassium carbonate and 35mL DM...
Embodiment 2
[0065] A synthetic method of etoxazole hapten, comprising the steps of:
[0066] S1, providing intermediate 1, wherein the preparation method of intermediate 1 is: add 2,6-difluorobenzamide and 2-chloroacetaldehyde dimethyl acetal to a one-necked bottle, and stir for 5 minutes under ice-water bath conditions , then slowly add concentrated sulfuric acid to it, after the dropwise addition, react overnight at room temperature, after the reaction, pour the reaction solution into ice water, stir well, extract 2-3 times with ethyl acetate, combine the organic phases, organic After washing and concentrating, the phase is purified by column to obtain intermediate 1; wherein, the ratio of the amount of substances of the 2,6-difluorobenzamide, 2-chloroacetaldehyde dimethylacetal and concentrated sulfuric acid is 1:1.2: 10;
[0067] S2, intermediate 2 is provided, wherein, the preparation method of intermediate 2 is: add 0.03mol 3-tert-butylphenol, 0.3mol potassium carbonate and 35mL DM...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com