Phosphorus binding agent with MOFs structure and preparation method and application thereof
A phosphorus binder and reaction technology, applied in the field of phosphorus binders with MOFs structure and its preparation, to achieve the effects of reducing phosphorus content, good biocompatibility, and improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
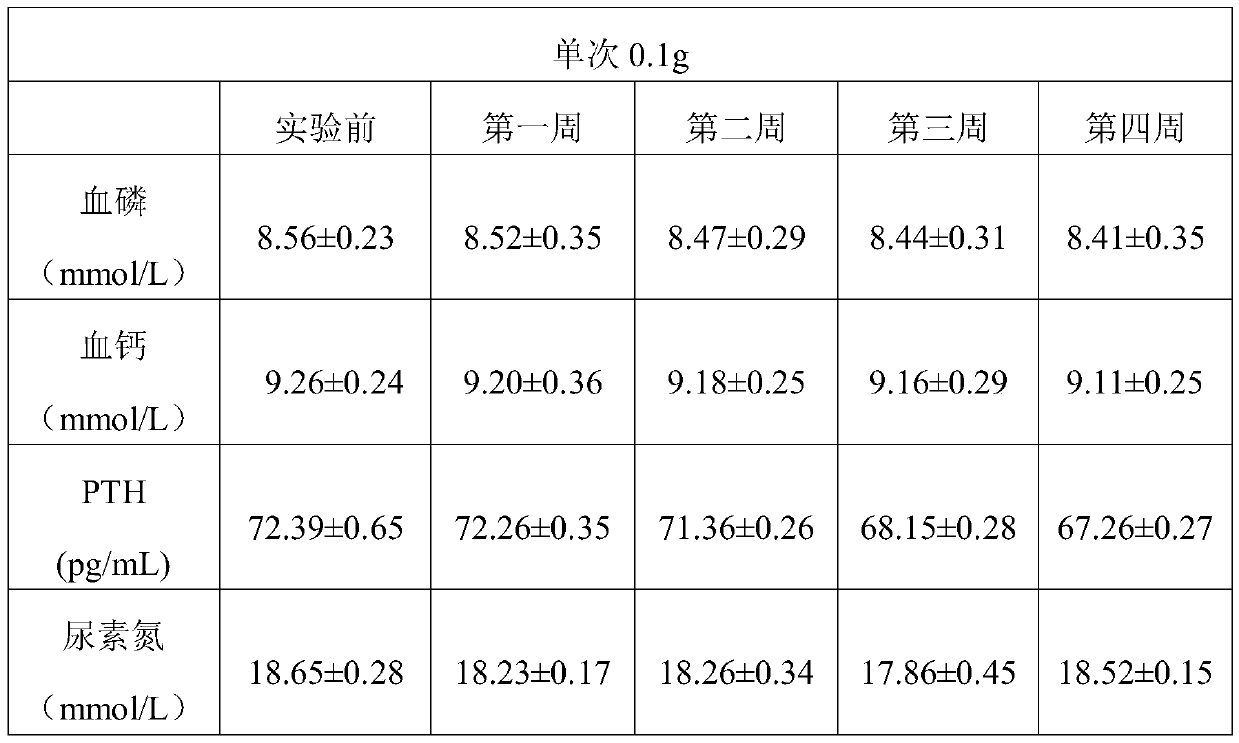
[0048] In this example, the phosphorus binder of MOFs structure was prepared by the following method
[0049] (1) In a polytetrafluoroethylene container, dissolve ferric chloride in deionized water, add trimesic acid and sodium acetate, and mix and react at 120°C for 24 hours, wherein the moles of ferric chloride, trimesic acid and sodium acetate The ratio is 1.5:1:2.5, centrifuged, washed with ethanol for 3 times, and freeze-dried to obtain Fe-MOFs with a particle size of 250nm;
[0050] (2) Disperse the obtained Fe-MOFs into a 70% ethanol solution with a mass fraction of 70%, the mass ratio of Fe-MOFs to ethanol solution is 1:2, add 2-hydroxypropyltrimethylammonium chloride, 2-hydroxypropyltrimethylammonium chloride The mass ratio of propyltrimethylammonium chloride to Fe-MOFs is 1:1.8, the pH value is adjusted to 6.5 with sodium hydroxide, heated to 75°C in a water bath, and after cationization reaction for 4 hours, it is centrifuged at a speed of 10000r / min 15min, washing...
Embodiment 2
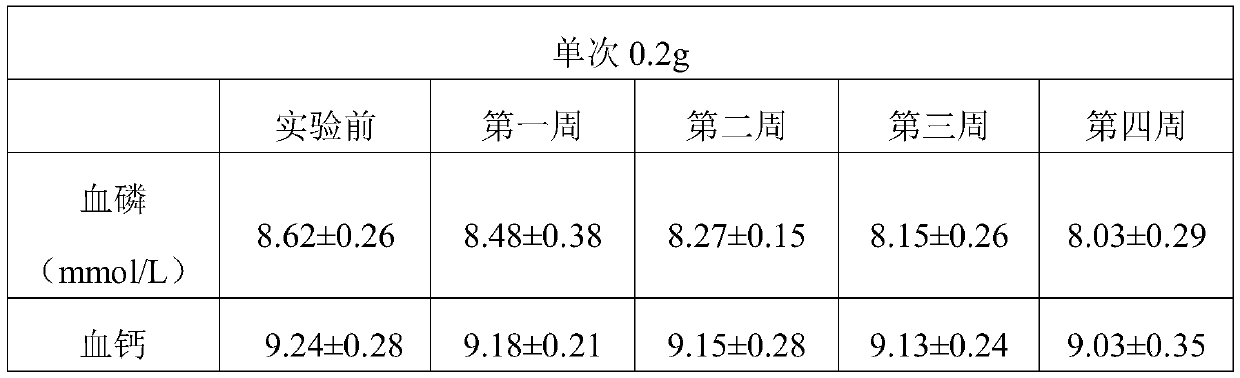
[0052] In this example, the phosphorus binder of MOFs structure was prepared by the following method
[0053] (1) In a polytetrafluoroethylene container, dissolve ferric sulfate in deionized water, add 2-aminotrimellitic acid and hydrochloric acid, and mix and react at 150°C for 20 hours, wherein ferric sulfate, 2-aminotrimellitic acid and hydrochloric acid The molar ratio is 2:1.5:3, centrifuged, washed 3 times with ethanol, and freeze-dried to obtain Fe-MOFs with a particle size of 200nm;
[0054] (2) Dispersing the obtained Fe-MOFs into a mass fraction of 75% ethanol solution, the mass ratio of Fe-MOFs to ethanol solution is 1:1.5, adding 2,3-epoxypropyltrimethylammonium chloride, The mass ratio of 2,3-epoxypropyltrimethylammonium chloride to Fe-MOFs is 1:1.5, the pH value is adjusted to 7 with sodium hydroxide, and the water bath is heated to 80°C. After cationization reaction for 4 hours, the speed Centrifuge at 12000r / min for 10min, wash and freeze-dry to obtain the Fe-...
Embodiment 3
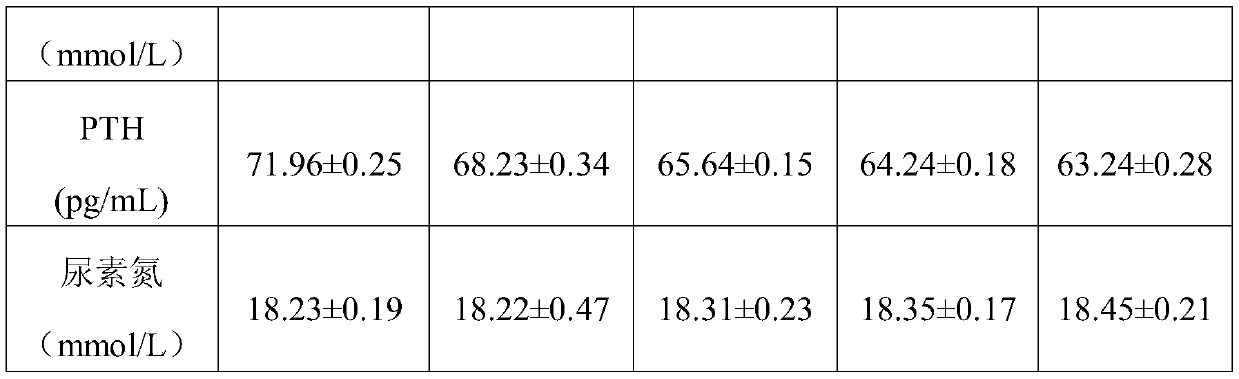
[0056] In this example, the phosphorus binder of MOFs structure was prepared by the following method
[0057] (1) In a polytetrafluoroethylene container, dissolve zinc nitrate in deionized water, add 2-aminoterephthalic acid and water, and mix and react at 110°C for 30 hours, wherein zinc nitrate, 2-aminoterephthalic acid and water The molar ratio is 1.6:1.2:2.7, centrifuged, washed with ethanol for 3 times, and freeze-dried to obtain Zn-MOFs with a particle size of 400nm;
[0058] (2) Disperse the obtained Zn-MOFs into a 65% ethanol solution by mass fraction, the mass ratio of Zn-MOFs to ethanol solution is 1:4, add 3-chloro-2-hydroxypropyl dodecyl dimethyl Ammonium chloride, the mass ratio of 3-chloro-2-hydroxypropyl dodecyl dimethyl ammonium chloride to Zn-MOFs is 1:2, the pH value is adjusted to 6 with sodium hydroxide, and heated to 70 in a water bath ℃, after cationization reaction for 5 hours, centrifugation at 8000r / min for 20 minutes, washing and freeze-drying to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com