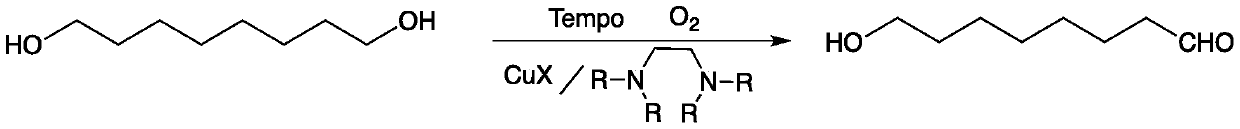
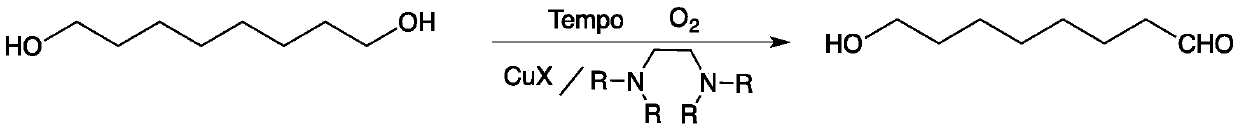
Method for preparing 8-hydroxyl caprylaldehyde
A technology of alkyl and octanediol, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc. It can solve the problems affecting the reaction and purification, and there will be double-sided acetylated by-products, so as to meet the reaction conditions Gentle, non-polluting yield effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
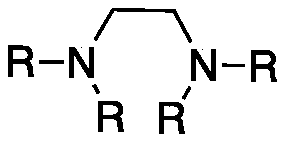
[0023] Preparation of composite catalyst (taking tetramethylethylenediamine and cuprous iodide as an example, the molar ratio is 1:1:1):
[0024] 30 grams (0.192mol) of Tempo, 22.3 grams (0.192mol) of tetramethylethylenediamine and 36.6 grams (0.192mol) of cuprous iodide were put into 70 milliliters of tetrahydrofuran solution and stirred for 1 hour, and the precipitate was filtered and at 45 °C after vacuum drying.
Embodiment 1
[0026] Add 5 grams (0.034mol) of 1,8-octanediol, 0.1 gram of composite catalyst (tetramethylethylenediamine, cuprous iodide and Tempo, molar ratio 1:1:1) and 20 mL of dry acetonitrile, the reaction mixture was stirred with air at room temperature, and the reaction was detected by TLC. After 24 hours of reaction, the reaction was completed, and the composite catalyst was removed by filtration (which can be recovered and reused), and the obtained filtrate was removed from the solvent. 4.65 g of liquid 8-hydroxyoctanal was obtained with a yield of 96.0% and a purity of 98.5% by gas phase analysis.
[0027] 1 HNMR (CDCl 3 , 400M), TMS): δ1.2-1.6 (m, 10H), 2.4 (t, 2H), 3.6 (t, 2H), 4.7 (s, br, 1H), 9.72 (s, 1H).
Embodiment 2
[0029] Add 10 grams (0.068mol) of 1,8-octanediol, 0.3 grams of composite catalyst (tetramethylethylenediamine, cuprous iodide and Tempo, molar ratio 1:1:1) and 40 mL of dry In acetonitrile, the reaction mixture was stirred with air at room temperature, and the reaction was detected by TLC. After reacting for 30 hours, the reaction was completed, and the composite catalyst was removed by filtration (which can be recycled), and the obtained filtrate was desolvated to obtain 9.5 grams of liquid 8-hydroxyoctanal, with a yield of 97.0% and a purity of 98.0% by gas phase analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com