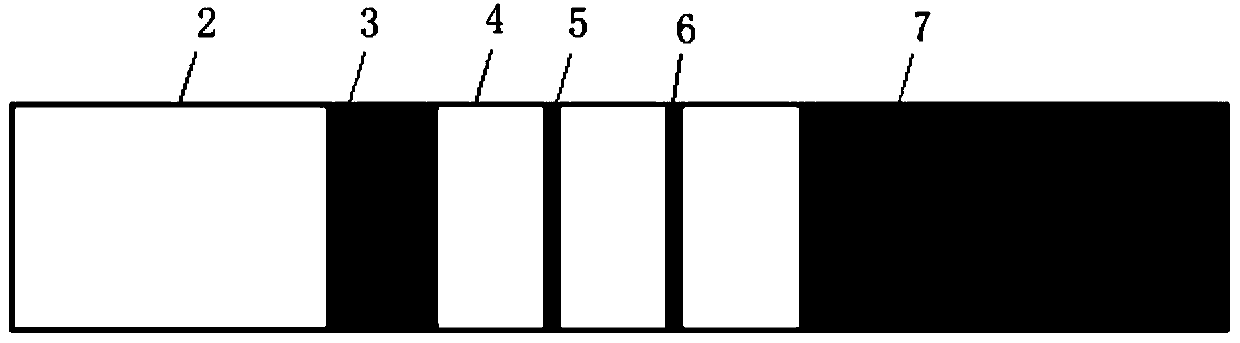
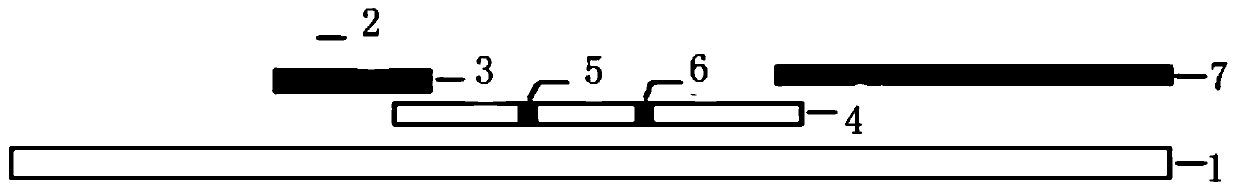
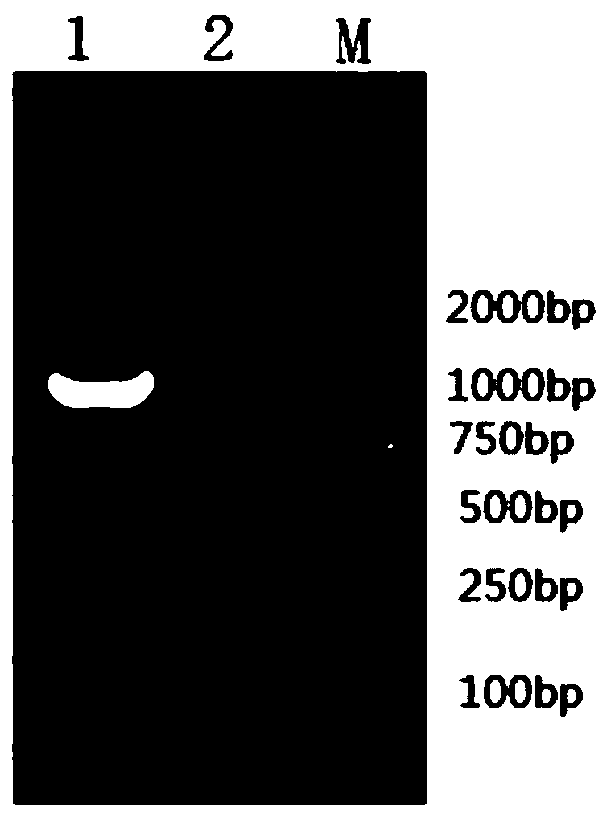
Rabies virus antibody quantitative detection kit as well as preparation method and detection method thereof
A technology for quantitative detection of rabies virus, applied in the field of rabies virus antibody quantitative detection kit and its preparation, can solve the problems of increased risk of rabies infection, potential safety hazards, insufficient protection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation and identification of rabies virus-like particles
[0066] (1) Extraction of viral genome
[0067] Rabies virus CTN-1 virus was inoculated into vero cells, and the virus liquid was collected 2 days after inoculation. According to Genbank's BABVCTN-1 strain genome sequence information (Accession NO.HQ317918.1), Primer5.0 software was used to design specific amplification G and M gene primers, primer sequences are shown in Table 1.
[0068] Table 1 Specific amplification primers for glycoprotein gene G and matrix protein M of rabies virus CTN-1 strain
[0069] serial number Primer name Sequence (5'-3') Restriction sites SEQ ID 3 CTNG-F CCC GAATTC ATGATTCCTCAAAGCTCTGTTGTTTG
EcoR I SEQ ID 4 CTNG-R TT TCTGCAG TTACAGCTTGGTCTCACCTCCG
Pst I SEQ ID 5 CTNM-F CCC CTCGAG ATGAACTTTCTACGCAAGATAGTGA
Xho I SEQ ID 6 CTNM-R CCC GGTACC CTATTCTAGGAGCAGGGAAGAGTC
Kpn I
[0070] Underli...
Embodiment 2
[0095] Preparation and identification of embodiment 2 rabies virus G protein monoclonal antibody
[0096](1) Select 5 female BALB / c mice aged 6-8 weeks, inject the aforementioned purified rabies virus G protein virus-like particles and the same amount of Freund's complete adjuvant emulsion into the mice, and inject 200 μl subcutaneously into each mouse . Two weeks later, the second immunization was carried out, and the mice were injected with rabies virus G protein virus-like particles and an equal amount of Freund's incomplete adjuvant emulsion, and the injection volume and method were the same as the first immunization. The third immunization was carried out two weeks after the second immunization, and the immunization method and virus injection volume were the same as the second immunization. Two weeks after the third immunization, when the serum ELISA titer reached 1:10000 or more after the tail-cut blood collection, the splenocytes and myeloma cells were removed at 6×10 ...
Embodiment 3
[0098] The preparation of embodiment 3 rabies virus antibody quantitative detection kit (colloidal gold)
[0099] (1) Preparation of sample pad
[0100] The purified rabies virus-like particles prepared in Example 1 were diluted to 1.0 mg / ml with 20 mM sodium tetraborate solution, containing 2% Tween-20, and sprayed on the glass cellulose membrane as a sample pad.
[0101] (2) Preparation of marker pads
[0102] With 0.2mol / L K 2 CO 3 After adjusting the pH value of the 25nm colloidal gold solution to 8.4, add the rabies virus-like particle monoclonal antibody to the colloidal gold solution with a protein concentration of 50 μg / ml after purification and rapid stirring And continue to stir for 30 minutes; add 10% BSA to a final concentration of 1%, stir for 30 minutes, centrifuge at 10,000r / min for 30 minutes, carefully suck off the supernatant, and the precipitate is the initially purified gold-labeled rabies virus-like particle. Cloning of antibody conjugates. Gold stand...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Protein concentration | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com