Palmatine hydrochloride-naringenin pharmaceutical eutectic crystal with slow-release effect
A technology of palmatine hydrochloride and naringenin, which is applied in the field of drug crystallization, can solve the problems that there are no public reports of palmatine hydrochloride and naringenin drugs, and achieve the effect of simple and easy preparation method and clear crystal structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 0.3 mmol of palmatine hydrochloride and 0.3 mmol of naringenin were completely dissolved in 200 mL of absolute ethanol to prepare a mixed solution; the above mixed solution was placed at room temperature and dried naturally to obtain Parmesan hydrochloride with sustained release Tin-naringenin drug co-crystal.
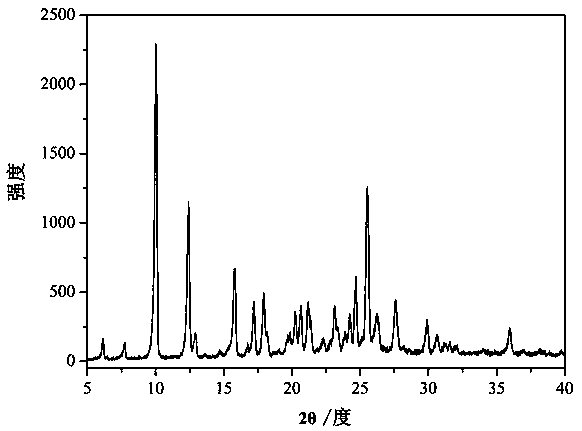
[0039] figure 1 It is the XRD figure of the palmatine hydrochloride-naringenin drug cocrystal prepared in the present embodiment. Depend on figure 1 It is known that the prepared drug co-crystal has a diffraction angle of 2 θ ° ± 0.2 for: 6.1, 7.7, 10.0, 12.4, 12.9, 13.6, 14.7, 15.3, 15.8, 16.7, 17.2, 17.9, 18.2, 19.0, 19.7, 19.8, 20.2, 20.6, 21.2, 21.3, 22.3, 22.8, 23.1 , 23.4, 23.9, 24.2, 24.7, 25.5, 26.2, 27.6, 29.9, 30.6, 31.2, 31.6, 32.0, 34.0, 36.0, 36.9 have characteristic diffraction peaks.
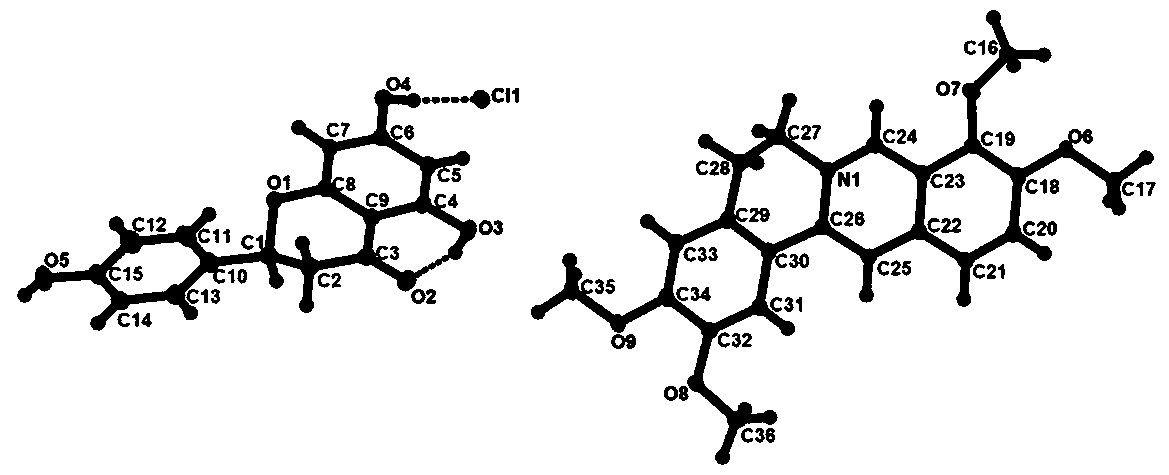
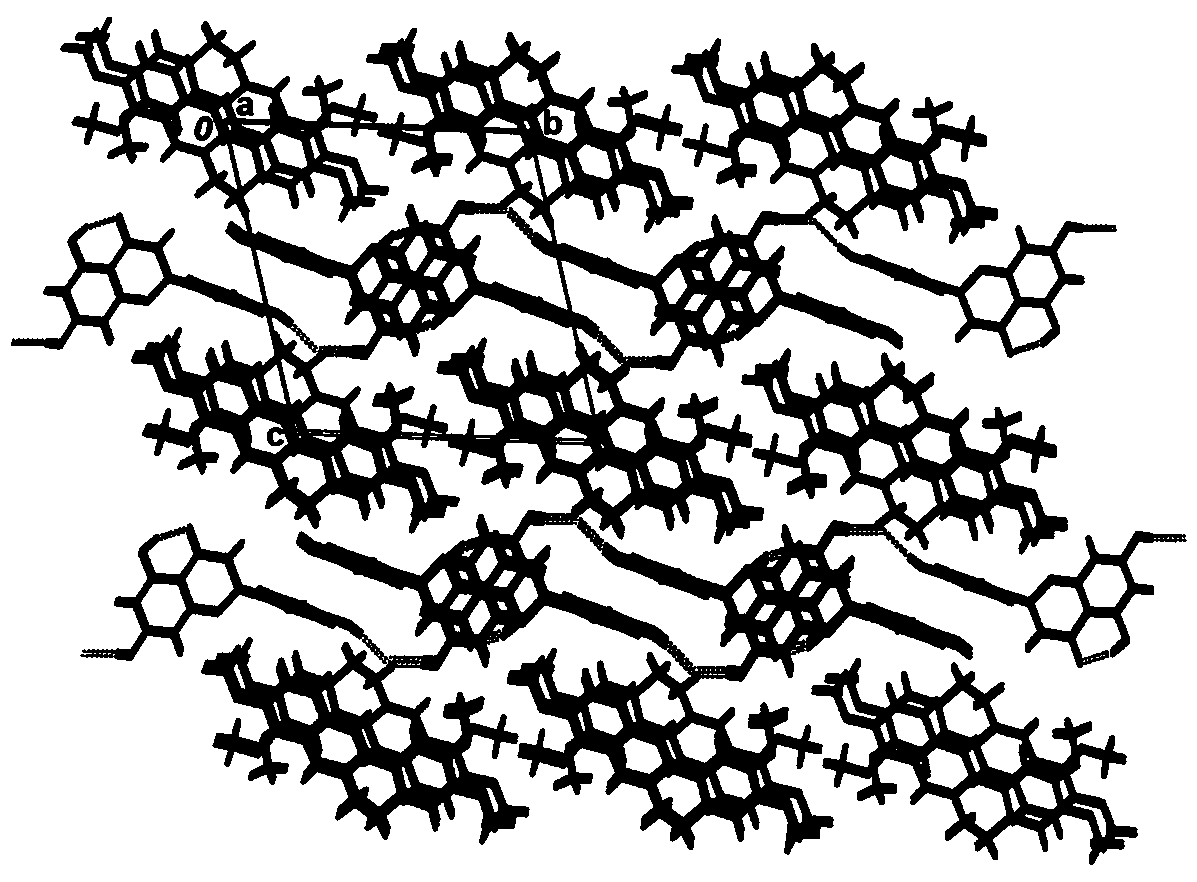
[0040] figure 2 It is the crystal structure unit of the palmatine hydrochloride-naringenin drug cocrystal prepared in this embodiment. Depend on figure 2...
Embodiment 2
[0050] Put 0.3 mmol of palmatine hydrochloride and 0.3 mmol of naringenin into 5 mL of absolute ethanol and mix, seal the beaker containing the mixture and stir at room temperature for 48 hours. The resulting precipitate is filtered, washed with a small amount of ethanol, and dried in the air A palmatine hydrochloride-naringenin co-crystal with sustained release is obtained.
[0051] Figure 11 It is the XRD figure of the palmatine hydrochloride-naringenin drug cocrystal prepared in the present embodiment. Depend on Figure 11 It is known that the palmatine hydrochloride-naringenin drug co-crystal prepared in this example and Example 1 have peaks at the same 2θ angle, indicating that the crystal structures of the two are the same.
Embodiment 3
[0053] Put 0.3 mmol of palmatine hydrochloride and 0.3 mmol of naringenin into 5 mL of methanol and mix, seal the beaker holding the mixture and stir at room temperature for 48 hours. The resulting precipitate is filtered, washed with a small amount of methanol, and dried to obtain A drug co-crystal of palmatine hydrochloride-naringenin with sustained release.
[0054] Figure 12 It is the XRD figure of the palmatine hydrochloride-naringenin drug cocrystal prepared in the present embodiment. Depend on Figure 12 It is known that the palmatine hydrochloride-naringenin drug co-crystal prepared in this example and Example 1 have peaks at the same 2θ angle, indicating that the crystal structures of the two are the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com