Lithium, aluminum and fluorine co-doped lithium iron phosphate positive electrode material and preparation method thereof
A technology of lithium iron phosphate and positive electrode materials, applied in the direction of positive electrodes, nanotechnology for materials and surface science, battery electrodes, etc., can solve the problems affecting the electrochemical performance and cycle performance of batteries, small tap density volumetric energy density, Problems such as low ion diffusion coefficient, achieve excellent charge-discharge cycle performance, improve electronic conductivity, and avoid side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
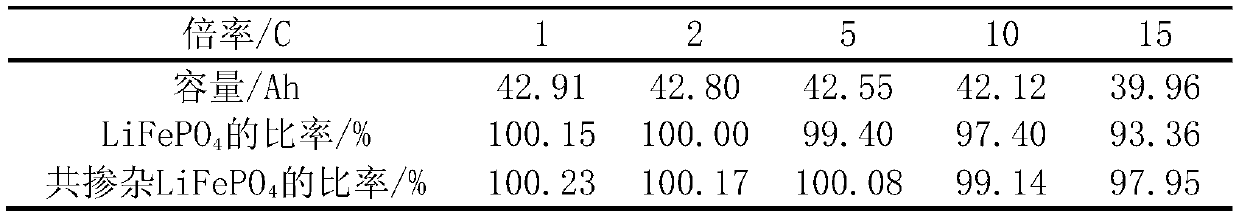
[0014] Reagent: NH 4 h 2 PO 4 , Fe(NO 3 ) 3 9H 2 O, Li 2 CO 3 , Al(NO 3 ) 3 9H 2 O, LiNO 3 , NH 4 F, glucose, deionized water and ethanol.
[0015] Preparation of lithium iron phosphate powder: 1.15g NH 4 h 2 PO 4 , 4.04g Fe(NO 3 ) 3 9H 2 O was dissolved in 60mL of deionized water, added 2g of glucose, and stirred for 30min. The reaction occurred, reacted at 140°C for 6h, cooled naturally to room temperature, and washed with water and ethanol three times. The samples were dried in an oven at 60 °C for 12 h, and then calcined at 500 °C for 10 h in a tube furnace under the protection of nitrogen. The calcined sample was mixed with 0.74g Li 2 CO 3 Perform ball milling and mixing for 2 hours, then calcinate at 700°C for 10 hours in a nitrogen atmosphere, grind after cooling, and sieve to remove iron to obtain LiFePO 4 sample.
[0016] Co-doped LiFePO with Li, Al and F 4 Preparation of: 0.0683gAl(NO 3 ) 3 9H 2 O, 0.0126 g LiNO 3 and 0.0283gNH 4 Dissolve...
Embodiment 2
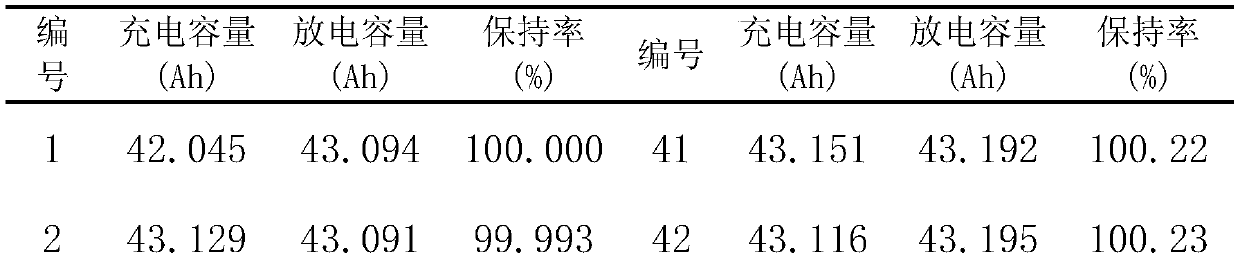
[0018] Preparation of lithium iron phosphate powder: 1.15gNH 4 h 2 PO 4 , 3.23gFe(NO 3 ) 3 9H 2 O was dissolved in 60ml deionized water, added 2.18 glucose, and stirred for 30min. The reaction occurred, reacted at 150°C for 8h, cooled naturally to room temperature, and washed with water and ethanol three times. The samples were dried in an oven at 80 °C for 14 h, and then calcined at 500 °C for 10 h in a tube furnace under the protection of nitrogen. The calcined sample was mixed with 0.81gLi 2 CO 3 Perform ball milling and mixing for 2 hours, then calcinate at 700°C for 10 hours in a nitrogen atmosphere, grind after cooling, and sieve to remove iron to obtain LiFePO 4 sample.
[0019] Co-doped LiFePO with Li, Al and F 4 Preparation of: 0.0750g Al(NO 3 ) 3 9H 2 O, 0.0138g LiNO 3 and 0.0296g NH 4 F was dissolved in deionized water and stirred for 1 h. Then add 1.14g LiFePO 4 The powder was stirred for 1 h. A reaction occurred and was kept at 160°C for 5h. Coo...
Embodiment 3
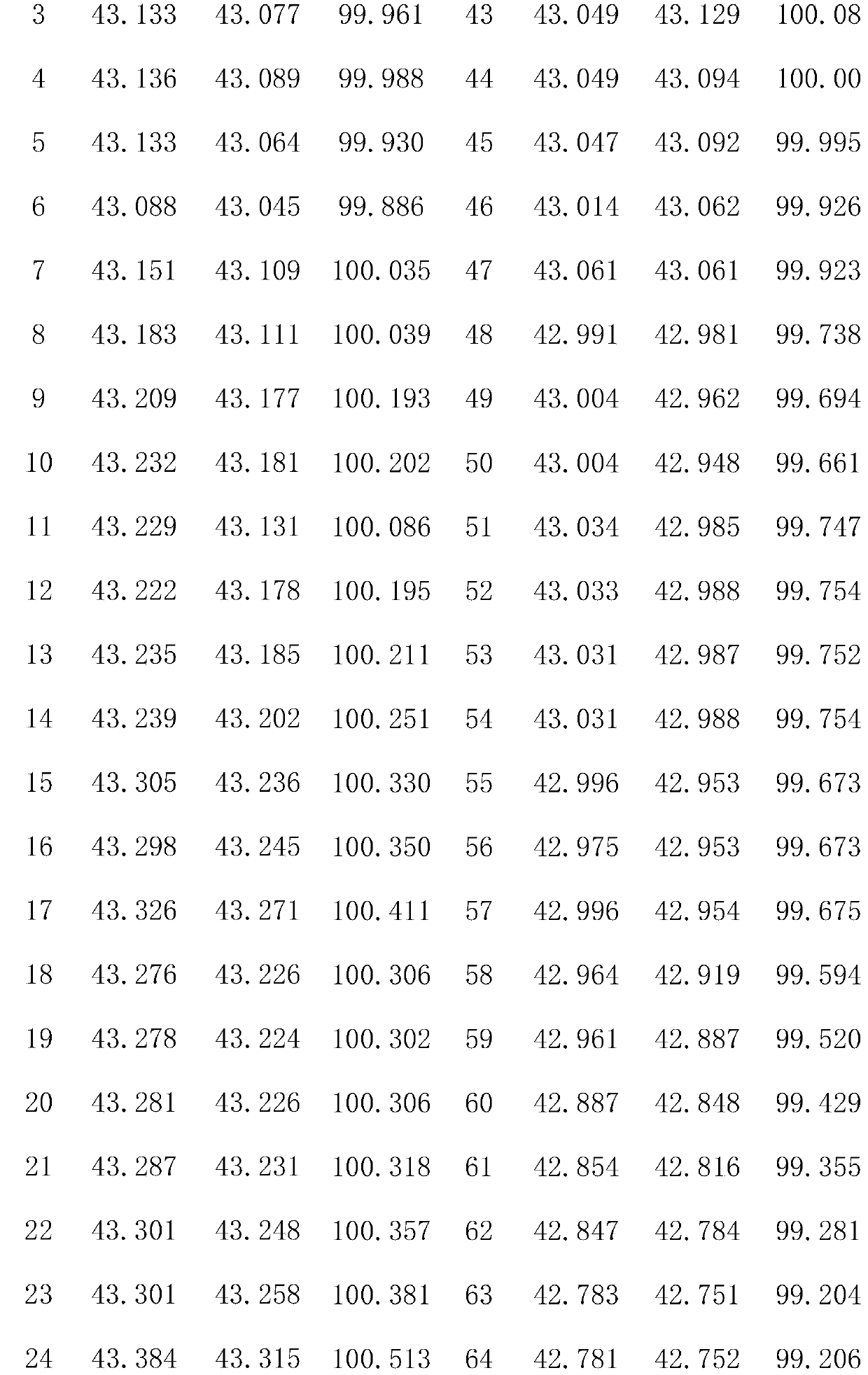
[0021] Preparation of lithium iron phosphate powder 1.15g NH 4 h 2 PO 4 , 4.84g Fe(NO 3 ) 3 9H 2 O was dissolved in 60 mL of deionized water, 1.82 g of glucose was added, and stirred for 30 min. The reaction occurred, reacted at 130°C for 8h, cooled naturally to room temperature, and washed with water and ethanol three times. The samples were dried in an oven at 70 °C for 13 h, and then calcined at 530 °C for 10 h in a tube furnace under the protection of argon. The calcined sample was mixed with 0.81g Li 2 CO 3 Perform ball milling and mixing for 2 hours, then calcinate at 700°C for 10 hours in a nitrogen atmosphere, grind after cooling, and sieve to remove iron to obtain LiFePO 4 sample.
[0022] Co-doped LiFePO with Li, Al and F 4 Preparation of: 0.0563g Al(NO 3 ) 3 9H 2 O, 0.0124 g LiNO 3 and 0.025g NH 4 Dissolve F in 30 mL deionized water and stir for 1 h. Then add 0.85g LiFePO 4The powder was stirred for 1 h. The mixture was transferred to a 50mL reacto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com